The Anatomy, Affinity, and Phylogenetic Significance of Markuelia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Systematics , Zoogeography , Andecologyofthepriapu
SYSTEMATICS, ZOOGEOGRAPHY, AND ECOLOGY OF THE PRIAPULIDA by J. VAN DER LAND (Rijksmuseum van Natuurlijke Historie, Leiden) With 89 text-figures and five plates CONTENTS Ι Introduction 4 1.1 Plan 4 1.2 Material and methods 5 1.3 Acknowledgements 6 2 Systematics 8 2.1 Historical introduction 8 2.2 Phylum Priapulida 9 2.2.1 Diagnosis 10 2.2.2 Classification 10 2.2.3 Definition of terms 12 2.2.4 Basic adaptive features 13 2.2.5 General features 13 2.2.6 Systematic notes 2 5 2.3 Key to the taxa 20 2.4 Survey of the taxa 29 Priapulidae 2 9 Priapulopsis 30 Priapulus 51 Acanthopriapulus 60 Halicryptus 02 Tubiluchidae 7° Tubiluchus 73 3 Zoogeography and ecology 84 3.1 Distribution 84 3.1.1 Faunistics 90 3.1.2 Expeditions 93 3.2 Ecology 96 3.2.1 Substratum 96 3.2.2 Depth 97 3.2.3 Temperature 97 3.24 Salinity 98 3.2.5 Oxygen 98 3.2.6 Food 99 3.2.7 Predators 100 3.2.8 Communities 102 3.3 Theoretical zoogeography 102 3.3.1 Bipolarity 102 3.3.2 Relict distribution 103 3.3.3 Concluding remarks 104 References 105 4 ZOOLOGISCHE VERHANDELINGEN 112 (1970) 1 INTRODUCTION The phylum Priapulida is only a very small group of marine worms but, since these animals apparently represent the last remnants of a once un- doubtedly much more important animal type, they are certainly of great scientific interest. Therefore, it is to be regretted that a comprehensive review of our present knowledge of the group does not exist, which does not only cause the faulty way in which the Priapulida are treated usually in textbooks and works on phylogeny, but also hampers further studies. -

Download Download
Dorjnamjaa et al. Mongolian Geoscientist 49 (2019) 41-49 https://doi.org/10.5564/mgs.v0i49.1226 Mongolian Geoscientist Review paper New scientific direction of the bacterial paleontology in Mongolia: an essence of investigation * Dorj Dorjnamjaa , Gundsambuu Altanshagai, Batkhuyag Enkhbaatar Department of Paleontology, Institute of Paleontology, Mongolian Academy of Sciences, Ulaanbaatar 15160, Mongolia *Corresponding author. Email: [email protected] ARTICLE INFO ABSTRACT Article history: We review the initial development of Bacterial Paleontology in Mongolia and Received 10 September 2019 present some electron microscopic images of fossil bacteria in different stages of Accepted 9 October 2019 preservation in sedimentary rocks. Indeed bacterial paleontology is one the youngest branches of paleontology. It has began in the end of 20th century and has developed rapidly in recent years. The main tasks of bacterial paleontology are detailed investigation of fossil microorganisms, in particular their morphology and sizes, conditions of burial and products of habitation that are reflected in lithological and geochemical features of rocks. Bacterial paleontology deals with fossil materials and is useful in analysis of the genesis of sedimentary rocks, and sedimentary mineral resources including oil and gas. The traditional paleontology is especially significant for evolution theory, biostratigraphy, biogeography and paleoecology; however bacterial paleontology is an essential first of all for sedimentology and for theories sedimentary ore genesis or biometallogeny Keywords: microfossils, phosphorite, sedimentary rocks, lagerstatten, biometallogeny INTRODUCTION all the microorganisms had lived and propagated Bacteria or microbes preserved well as fossils in without breakdowns. Bacterial paleontological various rocks, especially in sedimentary rocks data accompanied by the data on the first origin alike natural substances. -
![Autor: T-I Ij Ii 11 Iil U Li U Titel: H I] Il !J Band: I~ Ii Fj Ii [I S](https://docslib.b-cdn.net/cover/1235/autor-t-i-ij-ii-11-iil-u-li-u-titel-h-i-il-j-band-i-ii-fj-ii-i-s-221235.webp)
Autor: T-I Ij Ii 11 Iil U Li U Titel: H I] Il !J Band: I~ Ii Fj Ii [I S
;; ij Il 1I U it li ii H 13 u Autor: t-i ij ii 11 iil u li U Titel: H I] Il !J Band: I~ Ii fj iI [i s. 181 - 201. li 13 U li U It ii I,i Ii U t-i il il ii 11 il 11 li Il il lil U Ii il 1I l~ li Ii vörhanuen 1I 1,1 ti U n u 1I ii It 11 u I1 U li 1.1 it jj u ii u n 11 ii 11 H.l l,I II n il ii li ii ii U Ii U li 11 I] II !l !:! 1'1 I1 6 ?e> O$-15k~w'rl~1i. 180 T.A. Platonova& L. V. Kulangieva: Marine £noplido- ZOOSYST. ROSSICA Val. 3 @ Zoological Institute, Sl.Peter~ 1995 pharynx aod proposal of two new families. Zool. Tchesunov, A. V. 1991. On the slruclure of thc cephaJic Zhurn., 69(8): 5-17. (In Russian), culiclc in frcc-living ncmatodcsof thc famHy Linho- - Tchesunov, A.V. 1990b. Long-hairy Xyalida (Ncma- mocidae (Nemaloda. Chromadoria, Siphonolaimo- loda, Chromadoria, Monhystcrida) in thc Whitc ideaL Zoo!. Zhurn., 70(5): 21-27. (In RussianL The phylogeny and classification ofthe phylum Sca: ncw spccics, new combinations arid s~atusof Ih~ genus Trichotheristus. Zoo!. Zhurn., 69(10): 5-19. Reeei."ed 27 Mo)! /99J (In R!JssianL Cephalorhyncha A.V. Adrianov & V.V. Malakhov Adrianov, A.V. & Malakhoy, V. V. 1995. Thc phylogcny and c1assification of thc phylum Cephalorhyncha. Zoosystematica Rossica, 3(2),' 1994: 181-201. The phylum Ccphalorhyncha is a laxon including headproboscis worms: Priapulida, Loricifera, Kinorhyncha, Nematomorpha. -

Multi-Gene Analyses of the Phylogenetic Relationships Among the Mollusca, Annelida, and Arthropoda Donald J
Zoological Studies 47(3): 338-351 (2008) Multi-Gene Analyses of the Phylogenetic Relationships among the Mollusca, Annelida, and Arthropoda Donald J. Colgan1,*, Patricia A. Hutchings2, and Emma Beacham1 1Evolutionary Biology Unit, The Australian Museum, 6 College St. Sydney, NSW 2010, Australia 2Marine Invertebrates, The Australian Museum, 6 College St., Sydney, NSW 2010, Australia (Accepted October 29, 2007) Donald J. Colgan, Patricia A. Hutchings, and Emma Beacham (2008) Multi-gene analyses of the phylogenetic relationships among the Mollusca, Annelida, and Arthropoda. Zoological Studies 47(3): 338-351. The current understanding of metazoan relationships is largely based on analyses of 18S ribosomal RNA ('18S rRNA'). In this paper, DNA sequence data from 2 segments of 28S rRNA, cytochrome c oxidase subunit I, histone H3, and U2 small nuclear (sn)RNA were compiled and used to test phylogenetic relationships among the Mollusca, Annelida, and Arthropoda. The 18S rRNA data were included in the compilations for comparison. The analyses were especially directed at testing the implication of the Eutrochozoan hypothesis that the Annelida and Mollusca are more closely related than are the Annelida and Arthropoda and at determining whether, in contrast to analyses using only 18S rRNA, the addition of data from other genes would reveal these phyla to be monophyletic. New data and available sequences were compiled for up to 49 molluscs, 33 annelids, 22 arthropods, and 27 taxa from 15 other metazoan phyla. The Porifera, Ctenophora, and Cnidaria were used as the outgroup. The Annelida, Mollusca, Entoprocta, Phoronida, Nemertea, Brachiopoda, and Sipuncula (i.e., all studied Lophotrochozoa except for the Bryozoa) formed a monophyletic clade with maximum likelihood bootstrap support of 81% and a Bayesian posterior probability of 0.66 when all data were analyzed. -

Palaeobiology of the Early Ediacaran Shuurgat Formation, Zavkhan Terrane, South-Western Mongolia
Journal of Systematic Palaeontology ISSN: 1477-2019 (Print) 1478-0941 (Online) Journal homepage: http://www.tandfonline.com/loi/tjsp20 Palaeobiology of the early Ediacaran Shuurgat Formation, Zavkhan Terrane, south-western Mongolia Ross P. Anderson, Sean McMahon, Uyanga Bold, Francis A. Macdonald & Derek E. G. Briggs To cite this article: Ross P. Anderson, Sean McMahon, Uyanga Bold, Francis A. Macdonald & Derek E. G. Briggs (2016): Palaeobiology of the early Ediacaran Shuurgat Formation, Zavkhan Terrane, south-western Mongolia, Journal of Systematic Palaeontology, DOI: 10.1080/14772019.2016.1259272 To link to this article: http://dx.doi.org/10.1080/14772019.2016.1259272 Published online: 20 Dec 2016. Submit your article to this journal Article views: 48 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tjsp20 Download by: [Harvard Library] Date: 31 January 2017, At: 11:48 Journal of Systematic Palaeontology, 2016 http://dx.doi.org/10.1080/14772019.2016.1259272 Palaeobiology of the early Ediacaran Shuurgat Formation, Zavkhan Terrane, south-western Mongolia Ross P. Anderson a*,SeanMcMahona,UyangaBoldb, Francis A. Macdonaldc and Derek E. G. Briggsa,d aDepartment of Geology and Geophysics, Yale University, 210 Whitney Avenue, New Haven, Connecticut, 06511, USA; bDepartment of Earth Science and Astronomy, The University of Tokyo, 3-8-1 Komaba, Meguro, Tokyo, 153-8902, Japan; cDepartment of Earth and Planetary Sciences, Harvard University, 20 Oxford Street, Cambridge, Massachusetts, 02138, USA; dPeabody Museum of Natural History, Yale University, 170 Whitney Avenue, New Haven, Connecticut, 06511, USA (Received 4 June 2016; accepted 27 September 2016) Early diagenetic chert nodules and small phosphatic clasts in carbonates from the early Ediacaran Shuurgat Formation on the Zavkhan Terrane of south-western Mongolia preserve diverse microfossil communities. -

Worms, Nematoda
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 2001 Worms, Nematoda Scott Lyell Gardner University of Nebraska - Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Parasitology Commons Gardner, Scott Lyell, "Worms, Nematoda" (2001). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 78. https://digitalcommons.unl.edu/parasitologyfacpubs/78 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Published in Encyclopedia of Biodiversity, Volume 5 (2001): 843-862. Copyright 2001, Academic Press. Used by permission. Worms, Nematoda Scott L. Gardner University of Nebraska, Lincoln I. What Is a Nematode? Diversity in Morphology pods (see epidermis), and various other inverte- II. The Ubiquitous Nature of Nematodes brates. III. Diversity of Habitats and Distribution stichosome A longitudinal series of cells (sticho- IV. How Do Nematodes Affect the Biosphere? cytes) that form the anterior esophageal glands Tri- V. How Many Species of Nemata? churis. VI. Molecular Diversity in the Nemata VII. Relationships to Other Animal Groups stoma The buccal cavity, just posterior to the oval VIII. Future Knowledge of Nematodes opening or mouth; usually includes the anterior end of the esophagus (pharynx). GLOSSARY pseudocoelom A body cavity not lined with a me- anhydrobiosis A state of dormancy in various in- sodermal epithelium. -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -

Phylum Nemertea)
THE BIOLOGY AND SYSTEMATICS OF A NEW SPECIES OF RIBBON WORM, GENUS TUBULANUS (PHYLUM NEMERTEA) By Rebecca Kirk Ritger Submitted to the Faculty of the College of Arts and Sciences of American University in Partial Fulfillment of the Requirements for the Degree of Master of Science In Biology Chair: Dr. Qiristopher'Tudge m Dr.David C r. Jon L. Norenburg Dean of the College of Arts and Sciences JuK4£ __________ Date 2004 American University Washington, D.C. 20016 AMERICAN UNIVERSITY LIBRARY 1 1 0 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: 1421360 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. ® UMI UMI Microform 1421360 Copyright 2004 by ProQuest Information and Learning Company. All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest Information and Learning Company 300 North Zeeb Road P.O. Box 1346 Ann Arbor, Ml 48106-1346 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. THE BIOLOGY AND SYSTEMATICS OF A NEW SPECIES OF RIBBON WORM, GENUS TUBULANUS (PHYLUM NEMERTEA) By Rebecca Kirk Ritger ABSTRACT Most nemerteans are studied from poorly preserved museum specimens. -

Comparative Neuroanatomy of Mollusks and Nemerteans in the Context of Deep Metazoan Phylogeny
Comparative Neuroanatomy of Mollusks and Nemerteans in the Context of Deep Metazoan Phylogeny Von der Fakultät für Mathematik, Informatik und Naturwissenschaften der RWTH Aachen University zur Erlangung des akademischen Grades einer Doktorin der Naturwissenschaften genehmigte Dissertation vorgelegt von Diplom-Biologin Simone Faller aus Frankfurt am Main Berichter: Privatdozent Dr. Rudolf Loesel Universitätsprofessor Dr. Peter Bräunig Tag der mündlichen Prüfung: 09. März 2012 Diese Dissertation ist auf den Internetseiten der Hochschulbibliothek online verfügbar. Contents 1 General Introduction 1 Deep Metazoan Phylogeny 1 Neurophylogeny 2 Mollusca 5 Nemertea 6 Aim of the thesis 7 2 Neuroanatomy of Minor Mollusca 9 Introduction 9 Material and Methods 10 Results 12 Caudofoveata 12 Scutopus ventrolineatus 12 Falcidens crossotus 16 Solenogastres 16 Dorymenia sarsii 16 Polyplacophora 20 Lepidochitona cinerea 20 Acanthochitona crinita 20 Scaphopoda 22 Antalis entalis 22 Entalina quinquangularis 24 Discussion 25 Structure of the brain and nerve cords 25 Caudofoveata 25 Solenogastres 26 Polyplacophora 27 Scaphopoda 27 i CONTENTS Evolutionary considerations 28 Relationship among non-conchiferan molluscan taxa 28 Position of the Scaphopoda within Conchifera 29 Position of Mollusca within Protostomia 30 3 Neuroanatomy of Nemertea 33 Introduction 33 Material and Methods 34 Results 35 Brain 35 Cerebral organ 38 Nerve cords and peripheral nervous system 38 Discussion 38 Peripheral nervous system 40 Central nervous system 40 In search for the urbilaterian brain 42 4 General Discussion 45 Evolution of higher brain centers 46 Neuroanatomical glossary and data matrix – Essential steps toward a cladistic analysis of neuroanatomical data 49 5 Summary 53 6 Zusammenfassung 57 7 References 61 Danksagung 75 Lebenslauf 79 ii iii 1 General Introduction Deep Metazoan Phylogeny The concept of phylogeny follows directly from the theory of evolution as published by Charles Darwin in The origin of species (1859). -

Dornbos.Web.CV
Stephen Quinn Dornbos Associate Professor and Department Chair Department of Geosciences University of Wisconsin-Milwaukee Milwaukee, WI 53201-0413 Phone: (414) 229-6630 Fax: (414) 229-5452 E-mail: [email protected] http://uwm.edu/geosciences/people/dornbos-stephen/ EDUCATION 2003 Ph.D., Geological Sciences, University of Southern California, Los Angeles, CA. 1999 M.S., Geological Sciences, University of Southern California, Los Angeles, CA. 1997 B.A., Geology, The College of Wooster, Wooster, OH. ADDITIONAL EDUCATION 2002 University of Washington, Summer Marine Invertebrate Zoology Course, Friday Harbor Laboratories. 1997 Louisiana State University, Summer Field Geology Course. PROFESSIONAL EXPERIENCE 2017-Present Department Chair, Department of Geosciences, University of Wisconsin-Milwaukee. 2010-Present Associate Professor, Department of Geosciences, University of Wisconsin-Milwaukee. 2004-2010 Assistant Professor, Department of Geosciences, University of Wisconsin-Milwaukee. 2012-Present Adjunct Curator, Geology Department, Milwaukee Public Museum. 2004-Present Curator, Greene Geological Museum, University of Wisconsin- Milwaukee. 2003-2004 Postdoctoral Research Fellow, Department of Earth Sciences, University of Southern California. 2002 Research Assistant, Invertebrate Paleontology Department, Natural History Museum of Los Angeles County. EDITORIAL POSITIONS 2017-Present Editorial Board, Heliyon. 2015-Present Board of Directors, Coquina Press. 2014-Present Commentaries Editor, Palaeontologia Electronica. 2006-Present Associate Editor, Palaeontologia Electronica. Curriculum Vitae – Stephen Q. Dornbos 2 RESEARCH INTERESTS 1) Evolution and preservation of early life on Earth. 2) Evolutionary paleoecology of early animals during the Cambrian radiation. 3) Geobiology of microbial structures in Precambrian–Cambrian sedimentary rocks. 4) Cambrian reef evolution, paleoecology, and extinction. 5) Exceptional fossil preservation. HONORS AND AWARDS 2013 UWM Authors Recognition Ceremony. 2011 Full Member, Sigma Xi. -
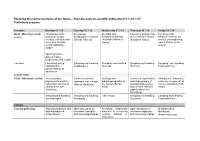
Exploring the Marine Meiofauna of the Azores – from Discovery to Scientific Publication (15.7.-24.7.19) Preliminary Program
Exploring the marine meiofauna of the Azores – from discovery to scientific publication (15.7.-24.7.19) Preliminary program: Schedule Monday 15.7.19 Tuesday 16.7.19 Wednesday 17.7.19 Thursday 18.7.19 Friday 19.7.19 09.00 - Morning session Introduction to the Meiofaunal Intertidal and Taxonomy and diversity The Proseriata Lecture workshop (people, Scalidophora (Andreas meiofaunal annelids of meiofaunal molluscs (Platyhelminthes): the facilities, schedule and Schmidt-Rhaesa) (Alejandro Martínez (Katharina Jörger) interest of unappealing aims; Ana Ricardo Garcia) worms (Marco Curini- Costa/ Katharina Galetti) Jörger) Opening lecture: Azores marine biodiversity (Ana Costa) Lab work Laboratory set-up, Sampling and handling Sampling and handling Sampling and handling Sampling and handling handling and Scalidophora Annelida Mollusca Platyhelminthes documentation of specimens LUNCH TIME 14.00 - Afternoon session Acoelomorpha – Marine nematodes: Geology and Taxonomy, systematics Rhabdocoel flatworms, phylogenetic position, taxonomy and ecology paleobiogeography of and biogeography of a diverse component of systematic and how to (Alberto Navarrete) the Azores (Sérgio meiofaunal Nemertea marine meiofauna (Tom identify them (Ulf Avila) and relevant research Artois) Jondelius) opportunities (Jon Norenburg) Sampling and handling Sampling and handling Lab session Sampling and handling Sampling and handling Acoelomorpha Nematoda Nemertea Platyhelminthes DINNER Evening gathering Sampling strategies and Open discussion on Adressing biodiversity Open discussion -

Exceptionally Preserved Fossil Assemblages Through Geologic Time and Space
Gondwana Research 48 (2017) 164–188 Contents lists available at ScienceDirect Gondwana Research journal homepage: www.elsevier.com/locate/gr GR Focus Review Exceptionally preserved fossil assemblages through geologic time and space A.D. Muscente a,⁎, James D. Schiffbauer b, Jesse Broce b, Marc Laflamme c, Kenneth O'Donnell d,ThomasH.Boage, Michael Meyer f, Andrew D. Hawkins g,JohnWarrenHuntleyb, Maria McNamara h, Lindsay A. MacKenzie i, George D. Stanley Jr. j, Nancy W. Hinman j, Michael H. Hofmann j, Shuhai Xiao g a Department of Earth and Planetary Sciences, Harvard University, Cambridge, MA 02138, USA b Department of Geological Sciences, University of Missouri, Columbia, MO 65211, USA c Department of Chemical and Physical Sciences, University of Toronto Mississauga, Mississauga, ON L5L 1C6, Canada d AECOM, Germantown, MD 20876, USA e Department of Geological Sciences, Stanford University, Stanford, CA 94305, USA f Geophysical Laboratory, Carnegie Institution for Science, Washington, DC 20015, USA g Department of Geosciences, Virginia Tech, Blacksburg, VA 24061, USA h School of Biological, Earth, and Environmental Sciences, University College Cork, Cork, T23 TK30, Ireland i Department of Geological Sciences, SUNY Geneseo, Geneseo, NY 14454, USA j Department of Geosciences, University of Montana, Missoula, MT 59812, USA article info abstract Article history: Geologic deposits containing fossils with remains of non-biomineralized tissues (i.e. Konservat-Lagerstätten) Received 23 February 2017 provide key insights into ancient organisms and ecosystems. Such deposits are not evenly distributed through Received in revised form 13 April 2017 geologic time or space, suggesting that global phenomena play a key role in exceptional fossil preservation.