3. ANDREAEACEAE Dumortier
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Revision of Schoenobryum (Cryphaeaceae, Bryopsida) in Africa1
Revision of Schoenobryum 147 Tropical Bryology 24: 147-159, 2003 A revision of Schoenobryum (Cryphaeaceae, Bryopsida) in Africa1 Brian J. O’Shea 141 Fawnbrake Avenue, London SE24 0BG, U.K. Abstract. The nine species and two varieties of Schoenobryum reported for Africa were investigated, and no characters were found that uniquely identified any of the taxa to be other than the pantropical Schoenobryum concavifolium. The following nine names become new synonyms of S. concavifolium: Cryphaea madagassa, C. subintegra, Acrocryphaea robusta, A. latifolia, A. subrobusta, A. tisserantii, A. latifolia var. microspora, A. plicatula and A. subintegra var. idanreense; a lectotype is selected for Acrocryphaea latifolia var. microspora P.de la Varde. INTRODUCTION as the majority have not been examined since the type description, and many have never been A recent checklist of Sub-Saharan Africa illustrated. (O’Shea, 1999) included nine species and two varieties of Schoenobryum, most of quite limited The purpose of this paper is to provide an distribution. Recent collecting in both Malawi overview of the genus worldwide, and to review (O’Shea et al., 2001) and Uganda (Wigginton et the taxonomic position of the African taxa. al., 2001) has shown the genus to be not uncommon, although there was only one CRYPHAEACEAE SCHIMP. 1856. previously published collection from the two countries (O’Shea, 1993). Apart from one Cryphaeaceae Schimp., Coroll. Bryol. Eur. 97. African taxon occurring in nine countries, the 1856 [‘1855’]. Type: Cryphaea D.Mohr in other 10 occurred in an average of 1.7 countries. F.Weber This particular profile is typical of unrevised genera in Africa, and indicative of a possible A brief review of the circumscription and need for revision (O’Shea, 1997), particularly systematics of the family, and the distinctions from related families (e.g. -

A Revised Red List of Bryophytes in Britain
ConservationNews Revised Red List distinguished from Extinct. This Red List uses Extinct in the Wild (EW) – a taxon is Extinct version 3.1 of the categories and criteria (IUCN, in the Wild when it is known to survive only in A revised Red List of 2001), along with guidelines produced to assist cultivation or as a naturalized population well with their interpretation and use (IUCN, 2006, outside the past range. There are no taxa in this 2008), further guidelines for using the system category in the British bryophyte flora. bryophytes in Britain at a regional level (IUCN, 2003), and specific Regionally Extinct (RE) – a taxon is regarded guidelines for applying the system to bryophytes as Regionally Extinct in Britain if there are no (Hallingbäck et al., 1995). post-1979 records and all known localities have Conservation OfficerNick Hodgetts presents the latest revised Red List for How these categories and criteria have been been visited and surveyed without success, or interpreted and applied to the British bryophyte if colonies recorded post-1979 are known to bryophytes in Britain. Dumortiera hirsuta in north Cornwall. Ian Atherton flora is summarized below, but anyone interested have disappeared. It should be appreciated that in looking into them in more depth should regional ‘extinction’ for bryophytes is sometimes he first published Red List of et al. (2001) and Preston (2010), varieties and consult the original IUCN documents, which less final than for other, more conspicuous bryophytes in Britain was produced subspecies have been disregarded. are available on the IUCN website (www. organisms. This may be because bryophytes are in 2001 as part of a Red Data Book 1980 has been chosen as the cut-off year to iucnredlist.org/technical-documents/categories- easily overlooked, or because their very efficient for bryophytes (Church et al., 2001). -

Plant Life MagillS Encyclopedia of Science
MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE Volume 4 Sustainable Forestry–Zygomycetes Indexes Editor Bryan D. Ness, Ph.D. Pacific Union College, Department of Biology Project Editor Christina J. Moose Salem Press, Inc. Pasadena, California Hackensack, New Jersey Editor in Chief: Dawn P. Dawson Managing Editor: Christina J. Moose Photograph Editor: Philip Bader Manuscript Editor: Elizabeth Ferry Slocum Production Editor: Joyce I. Buchea Assistant Editor: Andrea E. Miller Page Design and Graphics: James Hutson Research Supervisor: Jeffry Jensen Layout: William Zimmerman Acquisitions Editor: Mark Rehn Illustrator: Kimberly L. Dawson Kurnizki Copyright © 2003, by Salem Press, Inc. All rights in this book are reserved. No part of this work may be used or reproduced in any manner what- soever or transmitted in any form or by any means, electronic or mechanical, including photocopy,recording, or any information storage and retrieval system, without written permission from the copyright owner except in the case of brief quotations embodied in critical articles and reviews. For information address the publisher, Salem Press, Inc., P.O. Box 50062, Pasadena, California 91115. Some of the updated and revised essays in this work originally appeared in Magill’s Survey of Science: Life Science (1991), Magill’s Survey of Science: Life Science, Supplement (1998), Natural Resources (1998), Encyclopedia of Genetics (1999), Encyclopedia of Environmental Issues (2000), World Geography (2001), and Earth Science (2001). ∞ The paper used in these volumes conforms to the American National Standard for Permanence of Paper for Printed Library Materials, Z39.48-1992 (R1997). Library of Congress Cataloging-in-Publication Data Magill’s encyclopedia of science : plant life / edited by Bryan D. -

NEW DATA ABOUT MOSSES on the SVALBARD GLACIERS Olga
NEW DATA ABOUT MOSSES ON THE SVALBARD GLACIERS Olga Belkina Polar-Alpine Botanical Garden-Institute, Kola Science Center of the Russian Academy of Sciences, Apatity, Murmansk Province, Russia; e-mail: [email protected] Rapid melting and retreat of glaciers in the Arctic is a cause of sustainable long‐ In Svalbard moss populations were found on 9 glaciers. In 2012, during re‐examination of the populations a few term existence of ablation zone on them. Sometimes these areas are the habitats In 2007 B.R.Mavlyudov collected one specimen on individuals of Bryum cryophilum Mårtensson and some plants of some mosses partly due to good availability of water and cryoconite Bertilbreen (Paludella squarrosa (Hedw.) Brid.) and of Sanionia uncinata were found among H. polare shoots in substratum. 14 species were found in this unusual habitat on Alaska and Iceland: some specimens on Austre Grønfjordbreen (Ceratodon some large cushions. Therefore the next stage of cushion Andreaea rupestris Hedw., Ceratodon purpureus (Hedw.) Brid., Ditrichum purpureus (Hedw.) Brid., Warnstorfia sarmentosa succession had begun –emergence of a di‐ and multi‐species flexicaule (Schwaegr.) Hampe, Pohlia nutans (Hedw.) Lindb., Polytrichum (Wahlenb.) Hedenäs, Sanionia uncinata (Hedw.) community. A similar process was observed earlier on the juniperinum Hedw. (Benninghoff, 1955), Racomitrium fasciculare (Hedw.) Brid. Loeske, Hygrohypnella polare (Lindb.) Ignatov & bone of a mammal that was lying on the same glacier. (=Codriophorus fascicularis (Hedw.) Bendarek‐Ochyra et Ochyra) (Shacklette, Ignatova.). Ceratodon purpureus settled in center of almost spherical 1966), Drepanocladus berggrenii (C.Jens.) Broth. (Heusser, 1972), Racomitrium In 2009 populations of two latter species were studied cushion of Sanionia uncinata on the both butt‐ends of the crispulum var. -

Part 2 – Fruticose Species
Appendix 5.2-1 Vegetation Technical Appendix APPENDIX 5.2‐1 Vegetation Technical Appendix Contents Section Page Ecological Land Classification ............................................................................................................ A5.2‐1‐1 Geodatabase Development .............................................................................................. A5.2‐1‐1 Vegetation Community Mapping ..................................................................................... A5.2‐1‐1 Quality Assurance and Quality Control ............................................................................ A5.2‐1‐3 Limitations of Ecological Land Classification .................................................................... A5.2‐1‐3 Field Data Collection ......................................................................................................... A5.2‐1‐3 Supplementary Results ..................................................................................................... A5.2‐1‐4 Rare Vegetation Species and Rare Ecological Communities ........................................................... A5.2‐1‐10 Supplementary Desktop Results ..................................................................................... A5.2‐1‐10 Field Methods ................................................................................................................. A5.2‐1‐16 Supplementary Results ................................................................................................... A5.2‐1‐17 Weed Species -

The Moss Flora of Britain and Ireland, SECOND EDITION
This page intentionally left blank The Moss Flora of Britain and Ireland This book describes and illustrates in detail the 763 species of mosses currently known to occur in the British Isles and incorporates the most up-to-date information available on classification and nomenclature, together with recent synonyms. The species descriptions provide information on frequency, ecology, geographical relationships and distribution, including information on protected species and those species at risk. For many species there are footnotes to aid identification. In addition to the species descriptions there are descriptions of families and genera and also introductory information on conservation, collection, preservation and examination of material, together with advice on using the keys. An artificial key to genera provides the only workable comprehensive key published in the English language. As a further aid to the user a list of English names for all British mosses is included, plus a comprehensive glossary and bibliography. This second edition incorporates the very considerable advances in knowledge of mosses made in the last quarter of the twentieth century. In this time eight species new to science have been described in Britain, 25 species not previously known in the British Isles have been discovered and taxonomic revisions have led to the addition of a further 51 species. Fourteen species have been removed, bringing the total number of species described to 763. Additionally, modern taxonomic methods have led to an increase in the number of genera from 175 to 214. This thoroughly updated and comprehensive Flora represents a unique resource for all those interested in this fascinating group of organisms TONY SMITH received undergraduate and postgraduate degrees from Lincoln College, Oxford, before embarking on a research and teaching career at the University Colleges of Swansea and Bangor. -

World Heritage Values and to Identify New Values
FLORISTIC VALUES OF THE TASMANIAN WILDERNESS WORLD HERITAGE AREA J. Balmer, J. Whinam, J. Kelman, J.B. Kirkpatrick & E. Lazarus Nature Conservation Branch Report October 2004 This report was prepared under the direction of the Department of Primary Industries, Water and Environment (World Heritage Area Vegetation Program). Commonwealth Government funds were contributed to the project through the World Heritage Area program. The views and opinions expressed in this report are those of the authors and do not necessarily reflect those of the Department of Primary Industries, Water and Environment or those of the Department of the Environment and Heritage. ISSN 1441–0680 Copyright 2003 Crown in right of State of Tasmania Apart from fair dealing for the purposes of private study, research, criticism or review, as permitted under the Copyright Act, no part may be reproduced by any means without permission from the Department of Primary Industries, Water and Environment. Published by Nature Conservation Branch Department of Primary Industries, Water and Environment GPO Box 44 Hobart Tasmania, 7001 Front Cover Photograph: Alpine bolster heath (1050 metres) at Mt Anne. Stunted Nothofagus cunninghamii is shrouded in mist with Richea pandanifolia scattered throughout and Astelia alpina in the foreground. Photograph taken by Grant Dixon Back Cover Photograph: Nothofagus gunnii leaf with fossil imprint in deposits dating from 35-40 million years ago: Photograph taken by Greg Jordan Cite as: Balmer J., Whinam J., Kelman J., Kirkpatrick J.B. & Lazarus E. (2004) A review of the floristic values of the Tasmanian Wilderness World Heritage Area. Nature Conservation Report 2004/3. Department of Primary Industries Water and Environment, Tasmania, Australia T ABLE OF C ONTENTS ACKNOWLEDGMENTS .................................................................................................................................................................................1 1. -

Total of 10 Pages Only May Be Xeroxed
CENTRE FOR NEWFOUNDLAND STUDIES TOTAL OF 10 PAGES ONLY MAY BE XEROXED (Without Author's Permission) ,, l • ...J ..... The Disjunct Bryophyte Element of the Gulf of St. Lawrence Region: Glacial and Postglacial Dispersal and Migrational Histories By @Rene J. Belland B.Sc., M.Sc. A thesis submitted to the School of Graduate Studies in partial fulfilment of the requirements for the degree of Doctor of Philosophy Department of Biology Memorial University of Newfoundland December, 1Q84 St. John's Newfoundland Abstract The Gulf St. Lawrence region has a bryophyte flora of 698 species. Of these 267 (38%) are disjunct to this region from western North America, eastern Asia, or Europe. The Gulf of St. Lawrence and eastern North American distributions of the disjuncts were analysed and their possible migrational and dispersal histories during and after the Last Glaciation (Wisconsin) examined. Based on eastern North American distribution patterns, the disjuncts fell into 22 sub elements supporting five migrational/ dispersal histories or combinations of these : (1) migration from the south, (2) migration from the north, (3) migration from the west, (4) survival in refugia, and (5) introduction by man. The largest groups of disjuncts had eastern North American distributions supporting either survival of bryophytes in Wisconsin ice-free areas of the Gulf of St. Lawrence or postglacial migration to the Gulf from the south. About 26% of the disjuncts have complex histories and their distributions support two histories. These may have migrated to the Gulf from the west and/or north, or from the west and/or survived glaciation in Gulf ice-free areas. -

Chapter 3-1 Sexuality: Sexual Strategies Janice M
Glime, J. M. and Bisang, I. 2017. Sexuality: Sexual Strategies. Chapt. 3-1. In: Glime, J. M. Bryophyte Ecology. Volume 1. 3-1-1 Physiological Ecology. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 2 April 2017 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 3-1 SEXUALITY: SEXUAL STRATEGIES JANICE M. GLIME AND IRENE BISANG TABLE OF CONTENTS Expression of Sex............................................................................................................................................... 3-1-2 Unisexual and Bisexual Taxa............................................................................................................................. 3-1-2 Sex Chromosomes....................................................................................................................................... 3-1-6 An unusual Y Chromosome........................................................................................................................ 3-1-7 Gametangial Arrangement.......................................................................................................................... 3-1-8 Origin of Bisexuality in Bryophytes ................................................................................................................ 3-1-11 Monoicy as a Derived/Advanced Character.............................................................................................. 3-1-11 Anthocerotophyta and Multiple Reversals............................................................................................... -
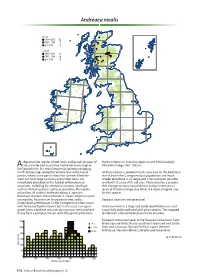
Andreaea Nivalis
Andreaea nivalis Britain 1990–2013 12 1950–1989 7 pre-1950 4 Ireland 1990–2013 0 1950–1989 0 pre-1950 0 characteristic species of wet rocks and gravels in areas of Nardia compressa, Scapania uliginosa and Pohlia ludwigii. Alate snow-lie and associated meltwater burns high in Altitudinal range: 880–1340 m. the Scottish hills. It is most frequently found on dripping, north-facing crags along the ‘cornice line’ at the top of Andreaea nivalis is abundant in its core area on the Ben Nevis corries, where snow persists into the summer. Here the massif and in the Cairngorms but populations are much moss can form large cushions and on Ben Nevis it is smaller elsewhere in its range and it has not been possible remarkably abundant in this habitat with numerous to refind it at some of its old sites. There must be a concern associates, including Deschampsia cespitosa, Saxifraga that changes in snow accumulation and persistence as a stellaris, Anthelia julacea, Lophozia opacifolia, Marsupella result of climate change may affect the more marginal sites sphacelata, M. stableri, Andreaea alpina, A. rupestris, for this species. Ditrichum zonatum, Kiaeria falcata, K. starkei, Polytrichastrum sexangulare, Racomitrium lanuginosum and, rarely, Dioicous; capsules are occasional. Oedipodium griffithianum. In the Cairngorms it often occurs with Andreaea frigida in burns but it also occurs on open Andreaea nivalis is a large and easily identifiable moss and gravel areas which are very wet during snow melt and here is not likely to be confused with other species. The mapped it may form a complex mosaic with Marsupella sphacelata, distribution is therefore believed to be accurate. -

THE ANDEAN SPECIES of PILEA by Ellsworth P. Killip INTRODUCTION
THE ANDEAN SPECIES OF PILEA By Ellsworth P. Killip INTRODUCTION Pilea is by far the largest genus of Urticaceae. Weddell in his final monograph 1 of the family recognized 159 species as valid, about 50 of which were said to occur in the Andes of South America. That these species had a very limited range of distribution was indicated by the fact that more than 30 were known from only a single collection and that only 9 of the others were in any sense widely distributed in the Andean countries. Two, P. microphyUa and P. pubescens, occurred throughout the American Tropics, and P. kyalina and P. dendrophUa extended eastward in South America. In the 50 years following the publication of this monograph only 10 species were described from the Andean region. Because of the large number of specimens of Pilea collected by expeditions to the Andes sponsored by the Smithsonian Institution, the New York Botanical Garden, the Gray Herbarium and the Arnold Arboretum of Harvard University, the Field Museum of Natural History, and the Academy of Natural Sciences, Philadelphia, which were submitted to me for identification but which could not be assigned to any known species, I began the preparation of a mono- graph of all the Andean species. This was completed in 1935 but unfortunately could not be printed in its entirety at that time. Instead, a greatly abridged paper was published,2 which contained a key to all the Andean species, descriptions of 30 new ones, and a few changes of name and of rank. In this there was no opportunity to present descriptions of the earlier species or to discuss their synonymy. -

Chapter 12 Productivity
Glime, J. M. 2017. Productivity. Chapt. 12. In: Glime, J. M. Bryophyte Ecology. Volume 1. Physiological Ecology. Ebook 12-1-1 sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 18 July 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 12 PRODUCTIVITY TABLE OF CONTENTS Productivity .......................................................................................................................................................... 12-2 Ecological Factors ................................................................................................................................................ 12-2 Ability to Invade ........................................................................................................................................... 12-2 Niche Differences ......................................................................................................................................... 12-3 Growth ................................................................................................................................................................. 12-3 Growth Measurements .................................................................................................................................. 12-4 Annual Length Increase ................................................................................................................................ 12-8 Uncoupling ...................................................................................................................................................