Chapter 12 Productivity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Translocation and Transport
Glime, J. M. 2017. Nutrient Relations: Translocation and Transport. Chapt. 8-5. In: Glime, J. M. Bryophyte Ecology. Volume 1. 8-5-1 Physiological Ecology. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 17 July 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 8-5 NUTRIENT RELATIONS: TRANSLOCATION AND TRANSPORT TABLE OF CONTENTS Translocation and Transport ................................................................................................................................ 8-5-2 Movement from Older to Younger Tissues .................................................................................................. 8-5-6 Directional Differences ................................................................................................................................ 8-5-8 Species Differences ...................................................................................................................................... 8-5-8 Mechanisms of Transport .................................................................................................................................... 8-5-9 Source to Sink? ............................................................................................................................................ 8-5-9 Enrichment Effects ..................................................................................................................................... 8-5-10 Internal Transport -

2.10 Meesia Longiseta HEDW. Code: 1389 Anhang: II
2.10 Meesia longiseta HEDW. Code: 1389 Anhang: II KLAUS WEDDELING, GERHARD LUDWIG & MONIKA HACHTEL, Bonn Namen: D: Langstieliges Schwanenhalsmoos, Langstieliges Meesemoos, Gestreckte Langborste E: Long-stalked Thread Moss, Long-shafted Swan Moss, F: – Systematik/Taxonomie: Bryophyta, Bryopsida, Bryidae, Splachnales, Meesiaceae. Synonyme: Amblyodon longisetus (HEDW.) P. BEAUV. Kennzeichen/Artbestimmung: Meesia longiseta ist ein 4–8 (–10) cm hohes, akrokarpes, unverzweigtes Laubmoos von grün-schwärzlicher Färbung. Die Art wächst in lockeren, weichen Rasen (Abb. 2.9). Das Stämmchen ist bis in die Spitze wurzelhaarig und im Moose Querschnitt dreikantig. Die Blättchen sind mehr oder weniger deutlich in 3 oder 6 Rei- hen angeordnet und vom Stämmchen abgespreizt. Die 2–3,5 mm langen, spitzen Blätt- chen laufen deutlich am Stämmchen herab, sind oberwärts gekielt, ganzrandig oder an der Spitze etwas gezähnt. Ihre deutlich entwickelte Rippe endet unterhalb der Blattspitze. Der Blattrand ist flach. Die Laminazellen sind rechteckig bis rhombisch und etwa 14 µm breit. Die rötlichen, gedrehten Seten der synözischen Art können über 10 cm lang wer- den. Die langbirnenförmige, aufrechte Kapsel hat einen deutlichen Hals. Bei der Spo- renreife im Juni und Juli werden die mit 36–44 µm Durchmesser recht großen Sporen frei- gesetzt. Die Chromosomenzahl ist nicht bekannt (FRITSCH 1991). Differenzierende Merkmale zu den ähnlichen Arten Meesia uliginosa und M. hexasticha sind der nicht ein- gerollte Blattrand, die kleineren Sporen und der Rippenquerschnitt mit kleinen, inneren Zellen (zusammengestellt nach CRUM & ANDERSON 1981, FRAHM 1979, LIMPRICHT 1895). Abbildungen der Art finden sich bei CRUM & ANDERSON (1981, Fig. 296, 297, S. 628, 629: Blättchen, Blattspitze, Habitus, Kapsel) und FRAHM (1979, Fig. -

NEW DATA ABOUT MOSSES on the SVALBARD GLACIERS Olga
NEW DATA ABOUT MOSSES ON THE SVALBARD GLACIERS Olga Belkina Polar-Alpine Botanical Garden-Institute, Kola Science Center of the Russian Academy of Sciences, Apatity, Murmansk Province, Russia; e-mail: [email protected] Rapid melting and retreat of glaciers in the Arctic is a cause of sustainable long‐ In Svalbard moss populations were found on 9 glaciers. In 2012, during re‐examination of the populations a few term existence of ablation zone on them. Sometimes these areas are the habitats In 2007 B.R.Mavlyudov collected one specimen on individuals of Bryum cryophilum Mårtensson and some plants of some mosses partly due to good availability of water and cryoconite Bertilbreen (Paludella squarrosa (Hedw.) Brid.) and of Sanionia uncinata were found among H. polare shoots in substratum. 14 species were found in this unusual habitat on Alaska and Iceland: some specimens on Austre Grønfjordbreen (Ceratodon some large cushions. Therefore the next stage of cushion Andreaea rupestris Hedw., Ceratodon purpureus (Hedw.) Brid., Ditrichum purpureus (Hedw.) Brid., Warnstorfia sarmentosa succession had begun –emergence of a di‐ and multi‐species flexicaule (Schwaegr.) Hampe, Pohlia nutans (Hedw.) Lindb., Polytrichum (Wahlenb.) Hedenäs, Sanionia uncinata (Hedw.) community. A similar process was observed earlier on the juniperinum Hedw. (Benninghoff, 1955), Racomitrium fasciculare (Hedw.) Brid. Loeske, Hygrohypnella polare (Lindb.) Ignatov & bone of a mammal that was lying on the same glacier. (=Codriophorus fascicularis (Hedw.) Bendarek‐Ochyra et Ochyra) (Shacklette, Ignatova.). Ceratodon purpureus settled in center of almost spherical 1966), Drepanocladus berggrenii (C.Jens.) Broth. (Heusser, 1972), Racomitrium In 2009 populations of two latter species were studied cushion of Sanionia uncinata on the both butt‐ends of the crispulum var. -

Kenai National Wildlife Refuge Species List, Version 2018-07-24
Kenai National Wildlife Refuge Species List, version 2018-07-24 Kenai National Wildlife Refuge biology staff July 24, 2018 2 Cover image: map of 16,213 georeferenced occurrence records included in the checklist. Contents Contents 3 Introduction 5 Purpose............................................................ 5 About the list......................................................... 5 Acknowledgments....................................................... 5 Native species 7 Vertebrates .......................................................... 7 Invertebrates ......................................................... 55 Vascular Plants........................................................ 91 Bryophytes ..........................................................164 Other Plants .........................................................171 Chromista...........................................................171 Fungi .............................................................173 Protozoans ..........................................................186 Non-native species 187 Vertebrates ..........................................................187 Invertebrates .........................................................187 Vascular Plants........................................................190 Extirpated species 207 Vertebrates ..........................................................207 Vascular Plants........................................................207 Change log 211 References 213 Index 215 3 Introduction Purpose to avoid implying -

Total of 10 Pages Only May Be Xeroxed
CENTRE FOR NEWFOUNDLAND STUDIES TOTAL OF 10 PAGES ONLY MAY BE XEROXED (Without Author's Permission) ,, l • ...J ..... The Disjunct Bryophyte Element of the Gulf of St. Lawrence Region: Glacial and Postglacial Dispersal and Migrational Histories By @Rene J. Belland B.Sc., M.Sc. A thesis submitted to the School of Graduate Studies in partial fulfilment of the requirements for the degree of Doctor of Philosophy Department of Biology Memorial University of Newfoundland December, 1Q84 St. John's Newfoundland Abstract The Gulf St. Lawrence region has a bryophyte flora of 698 species. Of these 267 (38%) are disjunct to this region from western North America, eastern Asia, or Europe. The Gulf of St. Lawrence and eastern North American distributions of the disjuncts were analysed and their possible migrational and dispersal histories during and after the Last Glaciation (Wisconsin) examined. Based on eastern North American distribution patterns, the disjuncts fell into 22 sub elements supporting five migrational/ dispersal histories or combinations of these : (1) migration from the south, (2) migration from the north, (3) migration from the west, (4) survival in refugia, and (5) introduction by man. The largest groups of disjuncts had eastern North American distributions supporting either survival of bryophytes in Wisconsin ice-free areas of the Gulf of St. Lawrence or postglacial migration to the Gulf from the south. About 26% of the disjuncts have complex histories and their distributions support two histories. These may have migrated to the Gulf from the west and/or north, or from the west and/or survived glaciation in Gulf ice-free areas. -

4.4 Biological Resources
4.4 BIOLOGICAL RESOURCES INTRODUCTION This section describes the existing biological resources that occur or have the potential to occur within the Project Area and vicinity. In addition, a description of applicable regulations is provided. The analysis evaluates the potential impacts to biological resources that could occur in association with the development of property in the commercial districts and the implementation of the Mobility Element. The Land Use Element/Zoning Code Amendments would modify the development regulations and no specific projects are proposed at this time. Likewise, the roadway and trail alignments are conceptual in nature. Therefore, the analysis is evaluated at a program‐level. With a programmatic study, such as this EIR, subsequent projects carried out under the proposed Land Use Element/ Zoning Code Amendments and Mobility Element Update may warrant site specific biological assessments and surveys once plans have been prepared. 1. ENVIRONMENTAL SETTING a. Regulatory Framework As part of the proposed Project’s review and approval there are a number of performance criteria and standard conditions that must be met. These include compliance with all of the terms, provisions, and requirements of applicable laws that relate to Federal, State, and local regulating agencies for impacts to biological resources. The following provides an overview of the applicable regulations with regard to the biological resources that may be present within the Project Area. (1) Federal (a) Migratory Bird Treaty Act The Migratory Bird Treaty Act (MBTA) protects individuals as well as any part, nest, or eggs of any bird listed as migratory. In practice, Federal permits issued for activities that potentially impact migratory birds typically have conditions that require pre‐disturbance surveys for nesting birds. -

Mosses: Weber and Wittmann, Electronic Version 11-Mar-00
Catalog of the Colorado Flora: a Biodiversity Baseline Mosses: Weber and Wittmann, electronic version 11-Mar-00 Amblystegiaceae Amblystegium Bruch & Schimper, 1853 Amblystegium serpens (Hedwig) Bruch & Schimper var. juratzkanum (Schimper) Rau & Hervey WEBER73B. Amblystegium juratzkanum Schimper. Calliergon (Sullivant) Kindberg, 1894 Calliergon cordifolium (Hedwig) Kindberg WEBER73B; HERMA76. Calliergon giganteum (Schimper) Kindberg Larimer Co.: Pingree Park, 2960 msm, 25 Sept. 1980, [Rolston 80114), !Hermann. Calliergon megalophyllum Mikutowicz COLO specimen so reported is C. richardsonii, fide Crum. Calliergon richardsonii (Mitten) Kindberg WEBER73B. Campyliadelphus (Lindberg) Chopra, 1975 KANDA75 Campyliadelphus chrysophyllus (Bridel) Kanda HEDEN97. Campylium chrysophyllum (Bridel) J. Lange. WEBER63; WEBER73B; HEDEN97. Hypnum chrysophyllum Bridel. HEDEN97. Campyliadelphus stellatus (Hedwig) Kanda KANDA75. Campylium stellatum (Hedwig) C. Jensen. WEBER73B. Hypnum stellatum Hedwig. HEDEN97. Campylophyllum Fleischer, 1914 HEDEN97 Campylophyllum halleri (Hedwig) Fleischer HEDEN97. Nova Guinea 12, Bot. 2:123.1914. Campylium halleri (Hedwig) Lindberg. WEBER73B; HERMA76. Hypnum halleri Hedwig. HEDEN97. Campylophyllum hispidulum (Bridel) Hedenäs HEDEN97. Campylium hispidulum (Bridel) Mitten. WEBER63,73B; HEDEN97. Hypnum hispidulum Bridel. HEDEN97. Cratoneuron (Sullivant) Spruce, 1867 OCHYR89 Cratoneuron filicinum (Hedwig) Spruce WEBER73B. Drepanocladus (C. Müller) Roth, 1899 HEDEN97 Nomen conserv. Drepanocladus aduncus (Hedwig) Warnstorf WEBER73B. -

Nová Bryologická Literatura X
34 Bryonora, Praha, 28 (2001) Liška J. et Pišút I. (2001): Invázne lišajníky. [Invasive lichens ] - Život, Prostr, Bratislava, 35: 98-99, Liška J, et Wild J. (2000): Závěrečná zpráva ze sledování lišejníků v České republice v období od května 1999 do dubna 2000, - 20 p., Tereza, Praha, Mayrhoher H., Lisická E, et Lackovičová A. (2001): New and interesting records of lichenized fiingi from Slovakia. - Biologia, Bratislava, 56: 355-361. McCarthy P.M., G. Kantvillas et Vězda A, (2001): Foliicolous lichens in Tasmania - Australasian Lichenol. 48: 16-26. Mucina L. et al. [incl. Pišút I ] (2000): Epiphytic lichen and moss vegetation along an altitude gradient on Mount Aenos (Kefalinia, Greece). - Biologia, Bratislava, 55: 43-48. Orthová V. et Kaňka R. (2001): Cladonia portentosa (lichenizované askomycéty) opáť nájdená na Slovensku. [Cladonia portentosa (lichenized Ascomycotina) recollected in Slovakia.] - Bull. Slov. Bot. Spoloč., Bratislava, 23: 29-32. Palice Z. (2001): Nová lichenologická literatura X, - Bryonora, Praha, 27: 23-28. Pišút I. (1999): Nachträge zur Kenntnis der Flechten der Slowakei 13. - Acta Rer. Natur. Mus. Nat. Slov., Bratislava, 45: 3-6. Pišút I. (2000): Nachträge zur Kenntnis der Flechten der Slowakei 14. - Acta Rer. Natur. Mus. Nat. Slov., Bratislava, 46: 11-14. Pišút I. (2000): Dobrá správa pre Bratislavu: Lišajníky sa vracajú! - Chrán. Územia Slov., Bratislava, 44: 3-5, Pišút I. (2001): RNDr. Ing. Antonín Vězda, CSc., octogenarian. - Biologia, Bratislava, 56: 458-460. Pišút I. et Kubinská A. (2000): Lišajníky a machorasty Prírodnej pamiatky Jajkovská suť. - Chrán. Üzemia Slov., Bratislava, 46: 35-36. Počubajová A., Guttová A. et Orthová V. (2000): Kaktuálnemu stavu lichenoflóry NP Slovenský raj. -

<I>Sphagnum</I> Peat Mosses
ORIGINAL ARTICLE doi:10.1111/evo.12547 Evolution of niche preference in Sphagnum peat mosses Matthew G. Johnson,1,2,3 Gustaf Granath,4,5,6 Teemu Tahvanainen, 7 Remy Pouliot,8 Hans K. Stenøien,9 Line Rochefort,8 Hakan˚ Rydin,4 and A. Jonathan Shaw1 1Department of Biology, Duke University, Durham, North Carolina 27708 2Current Address: Chicago Botanic Garden, 1000 Lake Cook Road Glencoe, Illinois 60022 3E-mail: [email protected] 4Department of Plant Ecology and Evolution, Evolutionary Biology Centre, Uppsala University, Norbyvagen¨ 18D, SE-752 36, Uppsala, Sweden 5School of Geography and Earth Sciences, McMaster University, Hamilton, Ontario, Canada 6Department of Aquatic Sciences and Assessment, Swedish University of Agricultural Sciences, SE-750 07, Uppsala, Sweden 7Department of Biology, University of Eastern Finland, P.O. Box 111, 80101, Joensuu, Finland 8Department of Plant Sciences and Northern Research Center (CEN), Laval University Quebec, Canada 9Department of Natural History, Norwegian University of Science and Technology University Museum, Trondheim, Norway Received March 26, 2014 Accepted September 23, 2014 Peat mosses (Sphagnum)areecosystemengineers—speciesinborealpeatlandssimultaneouslycreateandinhabitnarrowhabitat preferences along two microhabitat gradients: an ionic gradient and a hydrological hummock–hollow gradient. In this article, we demonstrate the connections between microhabitat preference and phylogeny in Sphagnum.Usingadatasetof39speciesof Sphagnum,withan18-locusDNAalignmentandanecologicaldatasetencompassingthreelargepublishedstudies,wetested -
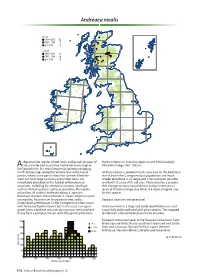
Andreaea Nivalis
Andreaea nivalis Britain 1990–2013 12 1950–1989 7 pre-1950 4 Ireland 1990–2013 0 1950–1989 0 pre-1950 0 characteristic species of wet rocks and gravels in areas of Nardia compressa, Scapania uliginosa and Pohlia ludwigii. Alate snow-lie and associated meltwater burns high in Altitudinal range: 880–1340 m. the Scottish hills. It is most frequently found on dripping, north-facing crags along the ‘cornice line’ at the top of Andreaea nivalis is abundant in its core area on the Ben Nevis corries, where snow persists into the summer. Here the massif and in the Cairngorms but populations are much moss can form large cushions and on Ben Nevis it is smaller elsewhere in its range and it has not been possible remarkably abundant in this habitat with numerous to refind it at some of its old sites. There must be a concern associates, including Deschampsia cespitosa, Saxifraga that changes in snow accumulation and persistence as a stellaris, Anthelia julacea, Lophozia opacifolia, Marsupella result of climate change may affect the more marginal sites sphacelata, M. stableri, Andreaea alpina, A. rupestris, for this species. Ditrichum zonatum, Kiaeria falcata, K. starkei, Polytrichastrum sexangulare, Racomitrium lanuginosum and, rarely, Dioicous; capsules are occasional. Oedipodium griffithianum. In the Cairngorms it often occurs with Andreaea frigida in burns but it also occurs on open Andreaea nivalis is a large and easily identifiable moss and gravel areas which are very wet during snow melt and here is not likely to be confused with other species. The mapped it may form a complex mosaic with Marsupella sphacelata, distribution is therefore believed to be accurate. -

New York Natural Heritage Program Rare Plant Status List May 2004 Edited By
New York Natural Heritage Program Rare Plant Status List May 2004 Edited by: Stephen M. Young and Troy W. Weldy This list is also published at the website: www.nynhp.org For more information, suggestions or comments about this list, please contact: Stephen M. Young, Program Botanist New York Natural Heritage Program 625 Broadway, 5th Floor Albany, NY 12233-4757 518-402-8951 Fax 518-402-8925 E-mail: [email protected] To report sightings of rare species, contact our office or fill out and mail us the Natural Heritage reporting form provided at the end of this publication. The New York Natural Heritage Program is a partnership with the New York State Department of Environmental Conservation and by The Nature Conservancy. Major support comes from the NYS Biodiversity Research Institute, the Environmental Protection Fund, and Return a Gift to Wildlife. TABLE OF CONTENTS Introduction.......................................................................................................................................... Page ii Why is the list published? What does the list contain? How is the information compiled? How does the list change? Why are plants rare? Why protect rare plants? Explanation of categories.................................................................................................................... Page iv Explanation of Heritage ranks and codes............................................................................................ Page iv Global rank State rank Taxon rank Double ranks Explanation of plant -

THE ANDEAN SPECIES of PILEA by Ellsworth P. Killip INTRODUCTION
THE ANDEAN SPECIES OF PILEA By Ellsworth P. Killip INTRODUCTION Pilea is by far the largest genus of Urticaceae. Weddell in his final monograph 1 of the family recognized 159 species as valid, about 50 of which were said to occur in the Andes of South America. That these species had a very limited range of distribution was indicated by the fact that more than 30 were known from only a single collection and that only 9 of the others were in any sense widely distributed in the Andean countries. Two, P. microphyUa and P. pubescens, occurred throughout the American Tropics, and P. kyalina and P. dendrophUa extended eastward in South America. In the 50 years following the publication of this monograph only 10 species were described from the Andean region. Because of the large number of specimens of Pilea collected by expeditions to the Andes sponsored by the Smithsonian Institution, the New York Botanical Garden, the Gray Herbarium and the Arnold Arboretum of Harvard University, the Field Museum of Natural History, and the Academy of Natural Sciences, Philadelphia, which were submitted to me for identification but which could not be assigned to any known species, I began the preparation of a mono- graph of all the Andean species. This was completed in 1935 but unfortunately could not be printed in its entirety at that time. Instead, a greatly abridged paper was published,2 which contained a key to all the Andean species, descriptions of 30 new ones, and a few changes of name and of rank. In this there was no opportunity to present descriptions of the earlier species or to discuss their synonymy.