The Use of Antibiotics in Food Producing Animals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
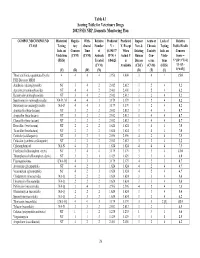
2002 FSIS National Residue Program, Section 4
Table 4.1 Scoring Table for Veterinary Drugs 2002 FSIS NRP, Domestic Monitoring Plan COMPOUND/COMPOUND Historical Regula- With- Relative Predicted Predicted Impact Acute or Lack of Relative CLASS Testing tory drawal Number V = V, Except New & Chronic Testing Public Health Info. on Concern Time of (0.19437* When Existing Toxicity Info. on Concern Violations (CVM) (CVM) Animals R*N) + Actual V Human Con- Viola- Score = (FSIS) Treated 0.84625 is Disease cerns tions V*[(D+3*T)/4] (CVM) Available (CDC) (CVM) (FSIS) *{1+[(L- (V) (R) (W) (N) (D) (T) (L) 1)*0.05]} Those antibiotics quantitated by the 4 4 4 4 3.956 4.000 3 4 1 15.0 FSIS Bioassay MRM Amikacin (aminoglycoside) NT 3 4 2 2.012 2.012 3 2 4 5.2 Apramycin (aminoglycoside) NT 4 4 2 2.401 2.401 3 2 4 6.2 Kanamycin (aminoglycoside) NT 3 4 2 2.012 2.012 3 2 4 5.2 Spectinomycin (aminoglycoside) NA-D, M 4 4 3 3.179 3.179 3 2 4 8.2 Streptomycin (aminoglycoside) NA-D 4 4 3 3.179 3.179 3 2 4 8.2 Amoxicillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Ampicillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Cloxacillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Hetacillin (beta-lactam) NT 2 2 2 1.624 1.624 3 4 4 7.0 Ticarcillin (beta-lactam) NT 2 2 2 1.624 1.624 3 4 4 7.0 Ceftiofur (cefalosporin) NT 3 2 3 2.596 2.596 4 2 4 7.5 Cefazolin (synthetic cefalosporin) NT 3 2 2 2.012 2.012 3 2 4 5.2 Chloramphenicol NA-N 4 2 1 1.624 1.624 4 4 4 7.5 Florfenicol (chloramphen. -

Cytosolic Selection Systems to Study Protein Stability
Cytosolic Selection Systems To Study Protein Stability Ajamaluddin Malik,* Antje Mueller-Schickert, James C. A. Bardwell Howard Hughes Medical Institute, Department of Molecular, Cellular and Developmental Biology, University of Michigan, Ann Arbor, Michigan, USA Here we describe biosensors that provide readouts for protein stability in the cytosolic compartment of prokaryotes. These bio- sensors consist of tripartite sandwich fusions that link the in vitro stability or aggregation susceptibility of guest proteins to the in vivo resistance of host cells to the antibiotics kanamycin, spectinomycin, and nourseothricin. These selectable markers confer Downloaded from antibiotic resistance in a wide range of hosts and are easily quantifiable. We show that mutations within guest proteins that af- fect their stability alter the antibiotic resistances of the cells expressing the biosensors in a manner that is related to the in vitro stabilities of the mutant guest proteins. In addition, we find that polyglutamine tracts of increasing length are associated with an increased tendency to form amyloids in vivo and, in our sandwich fusion system, with decreased resistance to aminoglycoside antibiotics. We demonstrate that our approach allows the in vivo analysis of protein stability in the cytosolic compartment with- out the need for prior structural and functional knowledge. http://jb.asm.org/ rotein folding has been intensely studied in vitro, providing us sion number WP_004614937). To eliminate expression of the toxic CcdB Pwith a detailed understanding of this process. However, the product present on this plasmid, we introduced a stop codon (TAA) by simplified conditions typically used in vitro (i.e., the use of single mutating the tyrosine at amino acid position 5 of the ccdB gene. -

2019-6654 Final Report of an Audit Carried out In
Ref. Ares(2019)7826731 - 19/12/2019 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Health and food audits and analysis DG(SANTE) 2019-6654 FINAL REPORT OF AN AUDIT CARRIED OUT IN BELARUS FROM 13 TO 24 MAY 2019 IN ORDER TO EVALUATE THE CONTROL OF RESIDUES AND CONTAMINANTS IN LIVE ANIMALS AND ANIMAL PRODUCTS INCLUDING CONTROLS ON VETERINARY MEDICINAL PRODUCTS Executive Summary This report describes the outcome of an audit carried out in Belarus from 13 to 24 May 2019 as part of the European Commission’s Directorate-General for Health and Food Safety planned work programme. The objective of the audit was to evaluate the effectiveness of official controls on residues and contaminants in live animals and animal products eligible for export to the European Union (EU). The audit assessed the implementation of the residue monitoring plan and also covered the authorisation, distribution and use of veterinary medicinal products, given that these areas have an impact on the monitoring of residues. Attention was also paid to examining the implementation of corrective actions indicated in response to specific recommendations made in the report of the previous residues audit to Belarus. The planning of the residue monitoring largely follows the principles of Directive 96/23/EC and covers for the most part an appropriate range of substances. The plan is nevertheless weakened by the fact that action levels for several substances across all commodities (including those for which listing has been requested) are not aligned with EU maximum residue limits, thus the plan would not be sufficient to demonstrate that commodities eligible for export to the EU would comply with such limits where they are lower that national limits. -

The Organic Chemistry of Drug Synthesis
The Organic Chemistry of Drug Synthesis VOLUME 2 DANIEL LEDNICER Mead Johnson and Company Evansville, Indiana LESTER A. MITSCHER The University of Kansas School of Pharmacy Department of Medicinal Chemistry Lawrence, Kansas A WILEY-INTERSCIENCE PUBLICATION JOHN WILEY AND SONS, New York • Chichester • Brisbane • Toronto Copyright © 1980 by John Wiley & Sons, Inc. All rights reserved. Published simultaneously in Canada. Reproduction or translation of any part of this work beyond that permitted by Sections 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful. Requests for permission or further information should be addressed to the Permissions Department, John Wiley & Sons, Inc. Library of Congress Cataloging in Publication Data: Lednicer, Daniel, 1929- The organic chemistry of drug synthesis. "A Wiley-lnterscience publication." 1. Chemistry, Medical and pharmaceutical. 2. Drugs. 3. Chemistry, Organic. I. Mitscher, Lester A., joint author. II. Title. RS421 .L423 615M 91 76-28387 ISBN 0-471-04392-3 Printed in the United States of America 10 987654321 It is our pleasure again to dedicate a book to our helpmeets: Beryle and Betty. "Has it ever occurred to you that medicinal chemists are just like compulsive gamblers: the next compound will be the real winner." R. L. Clark at the 16th National Medicinal Chemistry Symposium, June, 1978. vii Preface The reception accorded "Organic Chemistry of Drug Synthesis11 seems to us to indicate widespread interest in the organic chemistry involved in the search for new pharmaceutical agents. We are only too aware of the fact that the book deals with a limited segment of the field; the earlier volume cannot be considered either comprehensive or completely up to date. -

United States Patent (19) 11) Patent Number: 5,001,115 Sloan (45) Date of Patent: Mar
United States Patent (19) 11) Patent Number: 5,001,115 Sloan (45) Date of Patent: Mar. 19, 1991 54 PRODRUGS OF BIOLOGICALLY ACTIVE Primary Examiner-Mukund J. Shah HYDROXYAROMATIC COMPOUNDS Assistant Examiner-E. L. Ward Attorney, Agent, or Firm-Kerkam, Stowell, Kondracki (75 Inventor: Kenneth B. Sloan, Gainesville, Fla. & Clarke 73 Assignee: University of Florida, Gainesville, Fla, (57) ABSTRACT (21) Appl. No.: 352,919 Prodrugs of bio-active hydroxyaromatic drugs having 22 Filed: May 17, 1989 the structural formula: A pharmaceutically acceptable prodrug of a biologi (51] Int. Cl. ............... A61K 31/70; A61K 31/595; cally active, therapeutically effective hydroxyaromatic A61K 31/535; A61K 31/255 52 U.S. C. ..................................... 514/34: 514/289; drug, said prodrug being selected from the group con 514/169; 514/373; 514/222.8; 514/328; sisting of, (A) compounds having the structural for 514/360; 514/603; 514/417; 514/425; 514/518; mula: 546/44; 54.6/176; 546/75; 544/2; 536/64; 548/123; 548/209; 548/256; 548/417 DRUG-O-CR'R''-2) 58 Field of Search ............... 514/169,289, 373, 417, 514/425, 222.8, 328,360, 518, 603, 34; 536/64; wherein: 564/82, 155; 552/626; 546/44, 176, 75; 544/2; DRUG -O- is the hydroxyaromatic O-dehydro 548/123, 209, 256, 477, 595 residue of said drug; 56 References Cited R" and R' may be the same or different and may be H, PUBLICATIONS alkyl, aryl or electron withdrawing groups; 2 is a displaceable leaving group; and Katritzky, et al. J. Chem. Soc. -

Swedres-Svarm 2004
SVARM2004 Swedish Veterinary Antimicrobial Resistance Monitoring Preface .............................................................................................4 Summary ..........................................................................................6 Sammanfattning ...............................................................................7 Use of antimicrobials .........................................................................8 Resistance in zoonotic bacteria ......................................................12 Salmonella ..........................................................................................................12 Campylobacter ...................................................................................................15 Resistance in indicator bacteria ......................................................17 Escherichia coli ...................................................................................................17 Enterococcus .....................................................................................................20 Resistance in animal pathogens ......................................................28 Pig ......................................................................................................................28 Cattle ..................................................................................................................29 Horse ..................................................................................................................30 -

Danmap 2006.Pmd
DANMAP 2006 DANMAP 2006 DANMAP 2006 - Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark Statens Serum Institut Danish Veterinary and Food Administration Danish Medicines Agency National Veterinary Institute, Technical University of Denmark National Food Institute, Technical University of Denmark Editors: Hanne-Dorthe Emborg Danish Zoonosis Centre National Food Institute, Technical University of Denmark Mørkhøj Bygade 19 Contents DK - 2860 Søborg Anette M. Hammerum National Center for Antimicrobials and Contributors to the 2006 Infection Control DANMAP Report 4 Statens Serum Institut Artillerivej 5 DK - 2300 Copenhagen Introduction 6 DANMAP board: National Food Institute, Acknowledgements 6 Technical University of Denmark: Ole E. Heuer Frank Aarestrup List of abbreviations 7 National Veterinary Institute, Tecnical University of Denmark: Sammendrag 9 Flemming Bager Danish Veterinary and Food Administration: Summary 12 Justin C. Ajufo Annette Cleveland Nielsen Statens Serum Institut: Demographic data 15 Dominique L. Monnet Niels Frimodt-Møller Anette M. Hammerum Antimicrobial consumption 17 Danish Medicines Agency: Consumption in animals 17 Jan Poulsen Consumption in humans 24 Layout: Susanne Carlsson Danish Zoonosis Centre Resistance in zoonotic bacteria 33 Printing: Schultz Grafisk A/S DANMAP 2006 - September 2007 Salmonella 33 ISSN 1600-2032 Campylobacter 43 Text and tables may be cited and reprinted only with reference to this report. Resistance in indicator bacteria 47 Reprints can be ordered from: Enterococci 47 National Food Institute Escherichia coli 58 Danish Zoonosis Centre Tecnical University of Denmark Mørkhøj Bygade 19 DK - 2860 Søborg Resistance in bacteria from Phone: +45 7234 - 7084 diagnostic submissions 65 Fax: +45 7234 - 7028 E. -

U.S. National Residue Program: 2015 Residue Sampling Plans (Blue Book)
UNITED STATES National Residue Program for Meat, Poultry, and Egg Products 2015 Residue Sampling Plans United States Department of Agriculture Food Safety and Inspection Service Office of Public Health Science March 2015 Table of Contents Table of Contents ............................................................................................................................. ii Preface ............................................................................................................................................ iii Contacts and Comments ................................................................................................................. iii Acknowledgements ......................................................................................................................... iii Principal Authors ............................................................................................................................ iii Acronyms ........................................................................................................................................ iv Introduction ...................................................................................................................................... 1 Overview of the Sampling Plans ...................................................................................................... 5 Domestic Sampling Plan ..................................................................................................... 5 Import Reinspection Sampling -

The Effects of Combination Antibiotic Therapy on Methicillin-Resistant Staphylococcus Aureus Presented by Kasra Nick Fallah In
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UT Digital Repository The Effects of Combination Antibiotic Therapy on Methicillin-Resistant Staphylococcus aureus Presented by Kasra Nick Fallah in partial fulfillment of the requirements for completion of the Health Science Scholars honors program in the College of Natural Sciences at The University of Texas at Austin Spring 2018 Gregory C. Palmer, Ph.D. Date Supervising Professor Texas Institute for Discovery Education in Science Department of Medical Education Ruth Buskirk, Ph.D. Date Biology Honors Advisor Biological Sciences, College of Natural Sciences Table of Contents Abstract (3) Chapter 1: Literature Review (4) 1.1: The History of Antibiotics (4) 1.1.1: The Discovery of Penicillin (4) 1.1.2: The Different Classes of Antibiotics (7) 1.1.3: The Mechanisms of Action of Antibiotics (13) 1.2: Antibiotic Resistance (16) 1.2.1: The Rise of Antibiotic Resistance (16) 1.2.2: Mechanisms of Resistance (17) 1.2.3: How Antibiotic Resistance Spreads (20) 1.2.4: Methicillin-Resistant Staphylococcus aureus (21) 1.3: Streptomyces (24) 1.3.1: Selman Waksman (24) 1.3.2: Streptomyces: A Possible Solution to Antibiotic Resistance (25) 1.4: Combination Antibiotic Therapy (27) 1.4.1: The Positive/Negative Effects of Combination Antibiotic Therapy (27) 1.4.2: Drug Interactions (29) 1.4.3: Antibiotic Stewardship (30) Chapter 2: Research Manuscript (32) 2.1: Introduction (32) 1 2.2: Methods (35) 2.2.1: Strains, Media, and Growth Conditions (35) 2.2.2: Bacterial Strain Isolation and Identification (35) 2.2.3: Ethyl acetate Extractions (36) 2.2.4: Disc Assays (36) 2.3: Results & Discussion (38) 2.3.1: Isolation of Bacteria (38) 2.3.2: Organic Extractions (41) 2.3.3: Inhibition of MSSA and MRSA (41) 2.3.4: Inhibitory effect of the antibiotic produced by S. -

ARCH-Vet Anresis.Ch
Usage of Antibiotics and Occurrence of Antibiotic Resistance in Bacteria from Humans and Animals in Switzerland Joint report 2013 ARCH-Vet anresis.ch Publishing details © Federal Office of Public Health FOPH Published by: Federal Office of Public Health FOPH Publication date: November 2015 Editors: Federal Office of Public Health FOPH, Division Communicable Diseases. Elisabetta Peduzzi, Judith Klomp, Virginie Masserey Design and layout: diff. Marke & Kommunikation GmbH, Bern FOPH publication number: 2015-OEG-17 Source: SFBL, Distribution of Publications, CH-3003 Bern www.bundespublikationen.admin.ch Order number: 316.402.eng Internet: www.bag.admin.ch/star www.blv.admin.ch/gesundheit_tiere/04661/04666 Table of contents 1 Foreword 4 Vorwort 5 Avant-propos 6 Prefazione 7 2 Summary 10 Zusammenfassung 12 Synthèse 14 Sintesi 17 3 Introduction 20 3.1 Antibiotic resistance 20 3.2 About anresis.ch 20 3.3 About ARCH-Vet 21 3.4 Guidance for readers 21 4 Abbreviations 24 5 Antibacterial consumption in human medicine 26 5.1 Hospital care 26 5.2 Outpatient care 31 5.3 Discussion 32 6 Antibacterial sales in veterinary medicines 36 6.1 Total antibacterial sales for use in animals 36 6.2 Antibacterial sales – pets 37 6.3 Antibacterial sales – food producing animals 38 6.4 Discussion 40 7 Resistance in bacteria from human clinical isolates 42 7.1 Escherichia coli 42 7.2 Klebsiella pneumoniae 44 7.3 Pseudomonas aeruginosa 48 7.4 Acinetobacter spp. 49 7.5 Streptococcus pneumoniae 52 7.6 Enterococci 54 7.7 Staphylococcus aureus 55 Table of contents 1 8 Resistance in zoonotic bacteria 58 8.1 Salmonella spp. -

Guidelines for Atcvet Classification 2012
Guidelines for ATCvet classification 2012 ISSN 1020-9891 ISBN 978-82-8082-479-0 Suggested citation: WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATCvet classification 2012. Oslo, 2012. © Copyright WHO Collaborating Centre for Drug Statistics Methodology, Oslo, Norway. Use of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed. Guidelines for ATCvet classification 14th edition WHO Collaborating Centre for Drug Statistics Methodology Norwegian Institute of Public Health P.O.Box 4404 Nydalen N-0403 Oslo Norway Telephone: +47 21078160 Telefax: +47 21078146 E-mail: [email protected] Website: www.whocc.no Previous editions: 1992: Guidelines on ATCvet classification, 1st edition1) 1995: Guidelines on ATCvet classification, 2nd edition1) 1999: Guidelines on ATCvet classification, 3rd edition1) 2002: Guidelines for ATCvet classification, 4th edition2) 2003: Guidelines for ATCvet classification, 5th edition2) 2004: Guidelines for ATCvet classification, 6th edition2) 2005: Guidelines for ATCvet classification, 7th edition2) 2006: Guidelines for ATCvet classification, 8th edition2) 2007: Guidelines for ATCvet classification, 9th edition2) 2008: Guidelines for ATCvet classification, 10th edition2) 2009: Guidelines for ATCvet classification, 11th edition2) 2010: Guidelines for ATCvet classification, 12th edition2) 2011: Guidelines for ATCvet classification, 13th edition2) 1) Published by the Nordic Council on Medicines 2) Published by the WHO Collaborating Centre for Drug Statistics Methodology Preface The Anatomical Therapeutic Chemical classification system for veterinary medicinal products, ATCvet, has been developed by the Nordic Council on Medicines (NLN) in collaboration with the NLN’s ATCvet working group, consisting of experts from the Nordic countries. -

Nourseothricin N-Acetyl Transferase: a Positive Selection Marker for Mammalian Cells
Nourseothricin N-Acetyl Transferase: A Positive Selection Marker for Mammalian Cells Bose S. Kochupurakkal1, J. Dirk Iglehart1,2* 1 Department of Cancer Biology, Dana-Farber Cancer Institute; Boston, Massachusetts, United States of America, 2 Department of Surgery, Brigham and Women’s Hospital, Boston, Massachusetts, United States of America Abstract Development of Nourseothricin N-acetyl transferase (NAT) as a selection marker for mammalian cells is described. Mammalian cells are acutely susceptible to Nourseothricin, similar to the widely used drug Puromycin, and NAT allows for quick and robust selection of transfected/transduced cells in the presence of Nourseothricin. NAT is compatible with other selection markers puromycin, hygromycin, neomycin, blasticidin, and is a valuable addition to the repertoire of mammalian selection markers. Citation: Kochupurakkal BS, Iglehart JD (2013) Nourseothricin N-Acetyl Transferase: A Positive Selection Marker for Mammalian Cells. PLoS ONE 8(7): e68509. doi:10.1371/journal.pone.0068509 Editor: Graca Almeida-Porada, Wake Forest Institute for Regenerative Medicine, United States of America Received April 24, 2013; Accepted May 31, 2013; Published July 4, 2013 Copyright: ß 2013 Kochupurakkal, Iglehart. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: KSB is a recipient of the Teri Brodeur Fellowship and the Hale Fellowship. The work was supported by the Women’s Cancer Program at Dana-Farber Cancer Institute and National Cancer Institute Specialized Program in Research Excellence in Breast Cancer at Dana-Farber/Harvard Cancer Center (CA89393).