Arasites of Cattle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Age International Journal of Agricultural Research & Development
Title Code:-UPENG04282 VOL: 2, No: 1 Jan-June, 2018 NEW AGE INTERNATIONAL JOURNAL OF AGRICULTURAL RESEARCH & DEVELOPMENT NEW AGE MOBILIZATION NEW DELHI – 110043 (Registration No. - S/RS/SW/1420/2015) NEW AGE INTERNATIONAL JOURNAL OF AGRICULTURE RESEARCH AND DEVELOPMENT Halfyearly Published by : New Age Mobilization New Delhi -110043 REGISTRATION No. : S/RS/SW/1420/2015 Printed by : Pragati Press, Muzaffararnagar, U. P. Date of Publication : 12 Jan, 2018 Printing Place : Muzaffarnagar, U.P. On behalf of : Mrs. Jagesh Bhardwaj President, New Age Mobilization Published by : Mrs. Jagesh Bhardwaj President, New Age Mobilization EDITOR Dr. Tulsi Bhardwaj W. Scientist S.V. P. U. A. & T. Meerut, U.P. India Post Doctoral Fellow (Endeavour Award, Australia) NEW AGE INTERNATIONAL JOURNAL OF AGRICULTURE RESEARCH & DEVELOPMENT, Volume 2 Issue 1; 2018 NEW AGE INTERNATIONAL JOURNAL OF AGRICULTURE RESEARCH AND DEVELOPMENT Halfyearly Published by : New Age Mobilization, New Delhi-110043 (REGISTRATION No. - S/RS/SW/1420/2015 Eminent Members of Editorial board Dr. Rajendra Kumar Dr. Gadi V.P. Reddy Dr. Rajveer Singh Dr. Ashok Kumar Dr. Youva Raj Tyagi Director General Professor Dean Director Research Director & Head UPCAR Montana State University Colege of Veterinary Sc. S.V.P.U.A.& T GreenCem BV Lucknow ,U.P. India MT 59425, USA S.V.P.U.A. T,Meerut, U.P. Meerut U.P. India Netherland, Europe [email protected] [email protected] India [email protected] [email protected] www.upcaronline.org http://agresearch.monta [email protected] www.svbpmeerut.ac.in http://shineedge.in/about- www.iari.res.in na.edu m ceo www.svbpmeerut.ac.in www.researchgate.net/pro file/YouvaTyagi Dr. -

Incidence and Histopathological Study of Monieziosis in Goats of Jammu (J&K), India
Cibtech Journal of Zoology ISSN: 2319–3883 (Online) An Online International Journal Available at http://www.cibtech.org/cjz.htm 2013 Vol. 2 (1) January-April, pp.19-23/Mir et al. Research Article INCIDENCE AND HISTOPATHOLOGICAL STUDY OF MONIEZIOSIS IN GOATS OF JAMMU (J&K), INDIA *Muzaffar Rasool Mir1, M. Z. Chishti1, S. A. Dar1, Rajesh Katoch2, Majidah Rashid1, Fayaz Ahmad1, Hidayatullah Tak1 1Department of Zoology, the University of Kashmir Srinagar 190006 2Division of Veterinary Parasitology SKUAST-J R S Pura Jammu * Author for Correspondence ABSTRACT Necroscopic study of 284 goats was examined for Moniezia expansa Rudolphi, 1891 infection for the period of one year. The infection rate observed during the study was 2.11%. Histopathological study of the infected tissues with Moniezia expansa revealed shortened and flattened villi and local haemorrhages. The luminal site of the duodenum was found to b depressed like cavity because of Moniezia expansa. Key Words: Histopathology, Monieziasis, Goats, Jammu, Duodenum INTRODUCTION Goat rearing is a tribal profession of nomads (Bakerwals, Gaddies) and many other farming communities in Jammu and Kashmir. Goats contribute to the subsistence of small holders and landless rural poor. Goats due to improper management and unhygienic conditions are suffering from various parasitic diseases. Parasitic infection ranges from acute disease frequently with high rates of mortality and premature culling to subclinical infections, where goat may appear relatively healthy but perform below their potential. In broader sense, the factors dictating the level and extent of parasitism are climate, management conditions of pasture and animals, and the population dynamics of the parasites within the host and in the external environment. -
2002 FSIS National Residue Program, Section 4
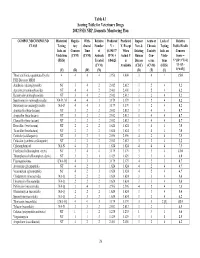
Table 4.1 Scoring Table for Veterinary Drugs 2002 FSIS NRP, Domestic Monitoring Plan COMPOUND/COMPOUND Historical Regula- With- Relative Predicted Predicted Impact Acute or Lack of Relative CLASS Testing tory drawal Number V = V, Except New & Chronic Testing Public Health Info. on Concern Time of (0.19437* When Existing Toxicity Info. on Concern Violations (CVM) (CVM) Animals R*N) + Actual V Human Con- Viola- Score = (FSIS) Treated 0.84625 is Disease cerns tions V*[(D+3*T)/4] (CVM) Available (CDC) (CVM) (FSIS) *{1+[(L- (V) (R) (W) (N) (D) (T) (L) 1)*0.05]} Those antibiotics quantitated by the 4 4 4 4 3.956 4.000 3 4 1 15.0 FSIS Bioassay MRM Amikacin (aminoglycoside) NT 3 4 2 2.012 2.012 3 2 4 5.2 Apramycin (aminoglycoside) NT 4 4 2 2.401 2.401 3 2 4 6.2 Kanamycin (aminoglycoside) NT 3 4 2 2.012 2.012 3 2 4 5.2 Spectinomycin (aminoglycoside) NA-D, M 4 4 3 3.179 3.179 3 2 4 8.2 Streptomycin (aminoglycoside) NA-D 4 4 3 3.179 3.179 3 2 4 8.2 Amoxicillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Ampicillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Cloxacillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Hetacillin (beta-lactam) NT 2 2 2 1.624 1.624 3 4 4 7.0 Ticarcillin (beta-lactam) NT 2 2 2 1.624 1.624 3 4 4 7.0 Ceftiofur (cefalosporin) NT 3 2 3 2.596 2.596 4 2 4 7.5 Cefazolin (synthetic cefalosporin) NT 3 2 2 2.012 2.012 3 2 4 5.2 Chloramphenicol NA-N 4 2 1 1.624 1.624 4 4 4 7.5 Florfenicol (chloramphen. -

Boselaphus Tragocamelus</I>
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- Published Research US Geological Survey 2008 Boselaphus tragocamelus (Artiodactyla: Bovidae) David M. Leslie Jr. U.S. Geological Survey, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/usgsstaffpub Leslie, David M. Jr., "Boselaphus tragocamelus (Artiodactyla: Bovidae)" (2008). USGS Staff -- Published Research. 723. https://digitalcommons.unl.edu/usgsstaffpub/723 This Article is brought to you for free and open access by the US Geological Survey at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USGS Staff -- Published Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. MAMMALIAN SPECIES 813:1–16 Boselaphus tragocamelus (Artiodactyla: Bovidae) DAVID M. LESLIE,JR. United States Geological Survey, Oklahoma Cooperative Fish and Wildlife Research Unit and Department of Natural Resource Ecology and Management, Oklahoma State University, Stillwater, OK 74078-3051, USA; [email protected] Abstract: Boselaphus tragocamelus (Pallas, 1766) is a bovid commonly called the nilgai or blue bull and is Asia’s largest antelope. A sexually dimorphic ungulate of large stature and unique coloration, it is the only species in the genus Boselaphus. It is endemic to peninsular India and small parts of Pakistan and Nepal, has been extirpated from Bangladesh, and has been introduced in the United States (Texas), Mexico, South Africa, and Italy. It prefers open grassland and savannas and locally is a significant agricultural pest in India. It is not of special conservation concern and is well represented in zoos and private collections throughout the world. DOI: 10.1644/813.1. -

Coinfection of Schistosoma (Trematoda) with Bacteria, Protozoa and Helminths
CHAPTER 1 Coinfection of Schistosoma (Trematoda) with Bacteria, Protozoa and Helminths ,† ‡ Amy Abruzzi* and Bernard Fried Contents 1.1. Introduction 3 1.2. Coinfection of Species of Schistosoma and Plasmodium 4 1.2.1. Animal studies 21 1.2.2. Human studies 23 1.3. Coinfection of Schistosoma Species with Protozoans other than in the Genus Plasmodium 24 1.3.1. Leishmania 32 1.3.2. Toxoplasma 32 1.3.3. Entamoeba 34 1.3.4. Trypanosoma 35 1.4. Coinfection of Schistosoma Species with Salmonella 36 1.4.1. Animal studies 36 1.4.2. Human studies 42 1.5. Coinfection of Schistosoma Species with Bacteria other than Salmonella 43 1.5.1. Mycobacterium 43 1.5.2. Helicobacter pylori 49 1.5.3. Staphylococcus aureus 50 1.6. Coinfection of Schistosoma and Fasciola Species 50 1.6.1. Animal studies 57 1.6.2. Human studies 58 * Skillman Library, Lafayette College, Easton, Pennsylvania, USA { Epidemiology, University of Medicine and Dentistry of New Jersey (UMDNJ), Piscataway, New Jersey, USA { Department of Biology, Lafayette College, Easton, Pennsylvania, USA Advances in Parasitology, Volume 77 # 2011 Elsevier Ltd. ISSN 0065-308X, DOI: 10.1016/B978-0-12-391429-3.00005-8 All rights reserved. 1 2 Amy Abruzzi and Bernard Fried 1.7. Coinfection of Schistosoma Species and Helminths other than the Genus Fasciola 59 1.7.1. Echinostoma 59 1.7.2. Hookworm 70 1.7.3. Trichuris 70 1.7.4. Ascaris 71 1.7.5. Strongyloides and Trichostrongyloides 72 1.7.6. Filarids 73 1.8. Concluding Remarks 74 References 75 Abstract This review examines coinfection of selected species of Schisto- soma with bacteria, protozoa and helminths and focuses on the effects of the coinfection on the hosts. -

The Role of Wild and Domestic Ungulates in Forming the Helminth Fauna of European Bison in Belarus
Sviatlana Polaz et al. European Bison Conservation Newsletter Vol 10 (2017) pp: 79–86 The role of wild and domestic ungulates in forming the helminth fauna of European bison in Belarus Sviatlana Polaz, Alena Anisimova, Palina Labanouskaya, Aksana Viarbitskaya, Vasili Kudzelich The State Research-Production Association “The Scientifically-Practical Centre of the National Academy of Sciences of Belarus for bio-resources”, Minsk, Belarus Abstract: Discussed is the role of wild and domestic ungulates in the formation of helminth fauna of the European bison in the Republic of Belarus. The current status of helminth infection of E. bison was determined and comparative analysis was conducted regarding the helminth fauna of other wild and domestic ungulates of the Republic of Belarus. Key words: European bison, helminth infection, Belarus Introduction The European bison (Bison bonasus) is a rare terrestrial mammal inhabiting a num- ber of countries including the territory of the Republic of Belarus. To facilitate fur- ther increase of its population, measures for conservation and sound management have been developed, aiming at preserving the already existing European bison population and enriching it with new individuals through an import of animals from other countries. One of present urgent problems in maintenance of European bison are parasitic infestations, since breeding programs carried out in Belarus concern not only the European bison but also other species of large mammals. Therefore an access to complete information about the types of helminths that are capable to affect the health of the E. bison and about factors that influence the formation of helmin- thiases is very important. One of these aspects is the transfer of helminths from one organism to another. -

Morphological and Molecular Studies of Moniezia Sp
RESEARCH PAPER Zoology Volume : 5 | Issue : 8 | August 2015 | ISSN - 2249-555X Morphological and Molecular Studies of Moniezia Sp. (Cestoda: Anaplocephalidea) A Parasite of the Domestic Goat Capra Hircus (L.) in Aurangabad District (M.S.), India. KEYWORDS Anaplocephalidea, Aurangabad, Capra hircus, India, Moniezia. Amol Thosar Ganesh Misal Department of Zoology, Dr. Babasaheb Ambedkar Department of Zoology, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad - 431004 Marathwada University, Aurangabad - 431004 Arun Gaware Sunita Borde Department of Zoology, Dr. Babasaheb Ambedkar Department of Zoology, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad-431004. Marathwada University, Aurangabad-431004. ABSTRACT Moniezia Sp.Nov. (Cestoda: Anaplocephalidea) is collected in the intestine of Capra hircus, Linnaeus, 1758 (Family: Bovidae) from Aurangabad district (M.S.), India. The present Cestode i.e. Moniezia Sp. Nov. differs other all known species is having the scolex almost squarish, mature proglottids nearly five times broader than long, Craspedote in shape, testes small in size, round to oval, 210-220 in numbers, cirrus pouch oval, ovary horse-shoe shaped, vitelline gland post ovarian.In molecular characterization of the parasites, the genomic DNA were amplified and sequenced. Based upon both morphological data and molecular analysis using bioinformatics tools, the Cestode is identified as confirmed to be representing Moniezia Sp. in mammalian host i.e. Goat. INTRODUCTION among individual orders. In addition to morphological The genus Moniezia was established by Blanchard, 1891. characters that are often variable, difficult to homologies, Skrjabin and Schulz (1937) divided this genus in to three molecular data have been widely used in phylogenetic subgenera as follows: studies of Cestodes generally and these Cestodes particu- larly using many genes and developed techniques as at- 1) Inter proglottidal glands grouped in rosettes--------------- tempts in solving many taxonomic problem. -

The Effect of Triaenophorus Nodulosus (Cestoda: Bothriocephalidea) Infection on Some Biochemical Parameters of the Liver of Perca fluviatilis
J Parasit Dis (Oct-Dec 2019) 43(4):566–574 https://doi.org/10.1007/s12639-019-01128-0 ORIGINAL ARTICLE The effect of Triaenophorus nodulosus (Cestoda: Bothriocephalidea) infection on some biochemical parameters of the liver of Perca fluviatilis 1 1 1 Ekaterina V. Borvinskaya • Irina V. Sukhovskaya • Lev P. Smirnov • 1 1 1 Albina A. Kochneva • Aleksey N. Parshukov • Marina Yu. Krupnova • 1 1 1 Elizaveta A. Buoy • Rimma U. Vysotskaya • Maria V. Churova Received: 2 February 2019 / Accepted: 29 May 2019 / Published online: 5 June 2019 Ó Indian Society for Parasitology 2019 Abstract Natural infection of 2 to 6-year-old perch with Keywords Helminth Á Triaenophorus Á Cestoda Á the cestode parasites Triaenophorus nodulosus was shown Perca fluviatilis Á Invasion Á Biochemical status to have minor effects on the studied components of the antioxidant defense system, nucleic acids degradation, and carbohydrate metabolism enzymes in the liver of the fish. Introduction The level of infection of 1–4 parasite larvae per fish observed in wild population of perch was shown to be The study of the effect of parasites on the biochemical moderate in terms of its effect on the health of the host fish. status of their host is important for clarifying the mutual The activity of hepatic enzymes b-galactosidase, b-glu- adaptations in the parasite–host system. A parasite directly cosidase, cathepsin D, and glutathione S-transferase affects its host by competing with it for resources; never- showed different responses in infected males and females, theless, there is usually a balance in the system, where which indicates different potential resistance of fish to the parasites cannot cause major damage to the host popula- stress exposure between genders. -

Waterborne Zoonotic Helminthiases Suwannee Nithiuthaia,*, Malinee T
Veterinary Parasitology 126 (2004) 167–193 www.elsevier.com/locate/vetpar Review Waterborne zoonotic helminthiases Suwannee Nithiuthaia,*, Malinee T. Anantaphrutib, Jitra Waikagulb, Alvin Gajadharc aDepartment of Pathology, Faculty of Veterinary Science, Chulalongkorn University, Henri Dunant Road, Patumwan, Bangkok 10330, Thailand bDepartment of Helminthology, Faculty of Tropical Medicine, Mahidol University, Ratchawithi Road, Bangkok 10400, Thailand cCentre for Animal Parasitology, Canadian Food Inspection Agency, Saskatoon Laboratory, Saskatoon, Sask., Canada S7N 2R3 Abstract This review deals with waterborne zoonotic helminths, many of which are opportunistic parasites spreading directly from animals to man or man to animals through water that is either ingested or that contains forms capable of skin penetration. Disease severity ranges from being rapidly fatal to low- grade chronic infections that may be asymptomatic for many years. The most significant zoonotic waterborne helminthic diseases are either snail-mediated, copepod-mediated or transmitted by faecal-contaminated water. Snail-mediated helminthiases described here are caused by digenetic trematodes that undergo complex life cycles involving various species of aquatic snails. These diseases include schistosomiasis, cercarial dermatitis, fascioliasis and fasciolopsiasis. The primary copepod-mediated helminthiases are sparganosis, gnathostomiasis and dracunculiasis, and the major faecal-contaminated water helminthiases are cysticercosis, hydatid disease and larva migrans. Generally, only parasites whose infective stages can be transmitted directly by water are discussed in this article. Although many do not require a water environment in which to complete their life cycle, their infective stages can certainly be distributed and acquired directly through water. Transmission via the external environment is necessary for many helminth parasites, with water and faecal contamination being important considerations. -

Mandrillus Leucophaeus Poensis)
Ecology and Behavior of the Bioko Island Drill (Mandrillus leucophaeus poensis) A Thesis Submitted to the Faculty of Drexel University by Jacob Robert Owens in partial fulfillment of the requirements for the degree of Doctor of Philosophy December 2013 i © Copyright 2013 Jacob Robert Owens. All Rights Reserved ii Dedications To my wife, Jen. iii Acknowledgments The research presented herein was made possible by the financial support provided by Primate Conservation Inc., ExxonMobil Foundation, Mobil Equatorial Guinea, Inc., Margo Marsh Biodiversity Fund, and the Los Angeles Zoo. I would also like to express my gratitude to Dr. Teck-Kah Lim and the Drexel University Office of Graduate Studies for the Dissertation Fellowship and the invaluable time it provided me during the writing process. I thank the Government of Equatorial Guinea, the Ministry of Fisheries and the Environment, Ministry of Information, Press, and Radio, and the Ministry of Culture and Tourism for the opportunity to work and live in one of the most beautiful and unique places in the world. I am grateful to the faculty and staff of the National University of Equatorial Guinea who helped me navigate the geographic and bureaucratic landscape of Bioko Island. I would especially like to thank Jose Manuel Esara Echube, Claudio Posa Bohome, Maximilliano Fero Meñe, Eusebio Ondo Nguema, and Mariano Obama Bibang. The journey to my Ph.D. has been considerably more taxing than I expected, and I would not have been able to complete it without the assistance of an expansive list of people. I would like to thank all of you who have helped me through this process, many of whom I lack the space to do so specifically here. -

Nematodes of the Genus Trichuris (Nematoda, Trichuridae), Parasitizing Sheep in Central and South-Eastern Regions of Ukraine
Vestnik Zoologii, 52(3): 193–204, 2018 DOI 10.2478/vzoo-2018-0020 UDC 595.132.6 NEMATODES OF THE GENUS TRICHURIS (NEMATODA, TRICHURIDAE), PARASITIZING SHEEP IN CENTRAL AND SOUTH-EASTERN REGIONS OF UKRAINE V. А. Yevstafi eva1, I. D. Yuskiv2, V. V. Melnychuk1, І. О. Yasnolob1, V. А. Kovalenko1, K. O. Horb1 1Poltava State Agrarian Academy, Skovorody st., 1/3, Poltava, 36003 Ukraine 2S. Z. Gzhytskiy National Veterinary and Biotech University of Lviv, Pekarska st., 50, Lviv, 79010 Ukraine E-mail: [email protected] Nematodes of the Genus Тrichuris (Nematoda, Trichuridae) Parasitizing Sheep in Central and South- Eastern Regions of Ukraine. Yevstafi eva, V. A., Yuskiv, I. D., Melnychuk, V. V., Yasnolob, I. O., Kovalenko, V. A., Horb, K. O. — Abundance and distribution of nematodes of the genus Тrichuris Schrank, 1788 parasitizing domestic sheep (Ovis aries Linnaeus, 1758) were studied in Poltava, Kyiv and Zaporizhzhia Regions of Ukraine. Th ree species of Тrichuris were found, Trichuris skrjabini Baskakov, 1924, Trichuris оvis Abildgaard, 1795 and Trichuris globulosa Linstow, 1901. Trichuris оvis and T. skrjabini were more common (54.9 and 35.7 %), whereas Т. globulosa was relatively rare (9.4 %) in the studied material. New species-specifi c and sex-related morphological characters and metric indices were reviewed as useful in better identifi cation of T. skrjabini, Т. оvis and Т. globulosa parasitizing sheep. Key words: Тrichuris, sheep, fauna, abundance, morphological characters, metric indices. Introduction Parasitic nematodes are one of most diverse and widely distributed group of parasitic worms. Th ey in- clude the economically important family Trichuridae Baird, 1853 with the monotypic genus Trichuris Schrank, 1788. -

WAAVP2019-Abstract-Book.Pdf
27th Conference of the World Association for the Advancement of Veterinary Parasitology JULY 7 – 11, 2019 | MADISON, WI, USA Dedicated to the legacy of Professor Arlie C. Todd Sifting and Winnowing the Evidence in Veterinary Parasitology @WAAVP2019 @WAAVP_2019 Abstract Book Joint meeting with the 64th American Association of Veterinary Parasitologists Annual Meeting & the 63rd Annual Livestock Insect Workers Conference WAAVP2019 27th Conference of the World Association for the Advancements of Veterinary Parasitology 64th American Association of Veterinary Parasitologists Annual Meeting 1 63rd Annualwww.WAAVP2019.com Livestock Insect Workers Conference #WAAVP2019 Table of Contents Keynote Presentation 84-89 OA22 Molecular Tools II 89-92 OA23 Leishmania 4 Keynote Presentation Demystifying 92-97 OA24 Nematode Molecular Tools, One Health: Sifting and Winnowing Resistance II the Role of Veterinary Parasitology 97-101 OA25 IAFWP Symposium 101-104 OA26 Canine Helminths II 104-108 OA27 Epidemiology Plenary Lectures 108-111 OA28 Alternative Treatments for Parasites in Ruminants I 6-7 PL1.0 Evolving Approaches to Drug 111-113 OA29 Unusual Protozoa Discovery 114-116 OA30 IAFWP Symposium 8-9 PL2.0 Genes and Genomics in 116-118 OA31 Anthelmintic Resistance in Parasite Control Ruminants 10-11 PL3.0 Leishmaniasis, Leishvet and 119-122 OA32 Avian Parasites One Health 122-125 OA33 Equine Cyathostomes I 12-13 PL4.0 Veterinary Entomology: 125-128 OA34 Flies and Fly Control in Outbreak and Advancements Ruminants 128-131 OA35 Ruminant Trematodes I Oral Sessions