2019-6654 Final Report of an Audit Carried out In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
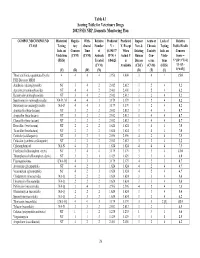
2002 FSIS National Residue Program, Section 4
Table 4.1 Scoring Table for Veterinary Drugs 2002 FSIS NRP, Domestic Monitoring Plan COMPOUND/COMPOUND Historical Regula- With- Relative Predicted Predicted Impact Acute or Lack of Relative CLASS Testing tory drawal Number V = V, Except New & Chronic Testing Public Health Info. on Concern Time of (0.19437* When Existing Toxicity Info. on Concern Violations (CVM) (CVM) Animals R*N) + Actual V Human Con- Viola- Score = (FSIS) Treated 0.84625 is Disease cerns tions V*[(D+3*T)/4] (CVM) Available (CDC) (CVM) (FSIS) *{1+[(L- (V) (R) (W) (N) (D) (T) (L) 1)*0.05]} Those antibiotics quantitated by the 4 4 4 4 3.956 4.000 3 4 1 15.0 FSIS Bioassay MRM Amikacin (aminoglycoside) NT 3 4 2 2.012 2.012 3 2 4 5.2 Apramycin (aminoglycoside) NT 4 4 2 2.401 2.401 3 2 4 6.2 Kanamycin (aminoglycoside) NT 3 4 2 2.012 2.012 3 2 4 5.2 Spectinomycin (aminoglycoside) NA-D, M 4 4 3 3.179 3.179 3 2 4 8.2 Streptomycin (aminoglycoside) NA-D 4 4 3 3.179 3.179 3 2 4 8.2 Amoxicillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Ampicillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Cloxacillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Hetacillin (beta-lactam) NT 2 2 2 1.624 1.624 3 4 4 7.0 Ticarcillin (beta-lactam) NT 2 2 2 1.624 1.624 3 4 4 7.0 Ceftiofur (cefalosporin) NT 3 2 3 2.596 2.596 4 2 4 7.5 Cefazolin (synthetic cefalosporin) NT 3 2 2 2.012 2.012 3 2 4 5.2 Chloramphenicol NA-N 4 2 1 1.624 1.624 4 4 4 7.5 Florfenicol (chloramphen. -

Dietary Exposure Assessment of Veterinary Antibiotics in Pork Meat on Children and Adolescents in Cyprus
foods Article Dietary Exposure Assessment of Veterinary Antibiotics in Pork Meat on Children and Adolescents in Cyprus Demetra Kyriakides 1,2,* , Andreas C. Lazaris 1, Konstantinos Arsenoglou 2, Maria Emmanouil 2, Olympia Kyriakides 3, Nikolaos Kavantzas 1 and Irene Panderi 4,* 1 Laboratory of Pathological Anatomy, Department of Clinical and Laboratory Medicine, School of Medicine, National and Kapodistrian University of Athens, 75, Mikras Asias Avenue, Goudi, 11527 Athens, Greece; [email protected] (A.C.L.); [email protected] (N.K.) 2 Veterinary Services, Ministry of Agriculture, Rural Development and Environment, 1417 Nicosia, Cyprus; [email protected] (K.A.); [email protected] (M.E.) 3 Archbishop Makarios III Hospital, 2012 Nicosia, Cyprus; [email protected] 4 Laboratory of Pharmaceutical Analysis, Panepistimiopolis, Division of Pharmaceutical Chemistry, Department of Pharmacy, National and Kapodistrian University of Athens, Zografou, 15771 Athens, Greece * Correspondence: [email protected] (D.K.); [email protected] (I.P.); Tel.: +30-210-727-4820 (I.P.) Received: 1 September 2020; Accepted: 13 October 2020; Published: 16 October 2020 Abstract: In recent years, huge amounts of antibiotics have been administered to farm animals, and as a result, residues of these antibiotics can accumulate in livestock products and, once consumed, may be transmitted to humans. Farm animals’ antibiotic treatment may therefore present a risk for consumers health, especially for children and adolescents. In children, the immune system is not fully developed, and thus, they are more susceptible than adults to resistant bacteria. A dietary exposure assessment was conducted on veterinary antibiotics found in raw pork meat among children and adolescents in Cyprus, since pork is the most consumed red meat in Cypriot population. -

The Ocean As a Global Reservoir of Antibiotic Resistance Genes
The Ocean as a Global Reservoir of Antibiotic Resistance Genes Stephen M. Hatosy,a Adam C. Martinya,b Department of Ecology and Evolutionary Biology, University of California, Irvine, California, USAa; Department of Earth System Science, University of California, Irvine, California, USAb Recent studies of natural environments have revealed vast genetic reservoirs of antibiotic resistance (AR) genes. Soil bacteria and Downloaded from human pathogens share AR genes, and AR genes have been discovered in a variety of habitats. However, there is little knowledge about the presence and diversity of AR genes in marine environments and which organisms host AR genes. To address this, we identified the diversity of genes conferring resistance to ampicillin, tetracycline, nitrofurantoin, and sulfadimethoxine in diverse marine environments using functional metagenomics (the cloning and screening of random DNA fragments). Marine environ- ments were host to a diversity of AR-conferring genes. Antibiotic-resistant clones were found at all sites, with 28% of the genes identified as known AR genes (encoding beta-lactamases, bicyclomycin resistance pumps, etc.). However, the majority of AR genes were not previously classified as such but had products similar to proteins such as transport pumps, oxidoreductases, and hydrolases. Furthermore, 44% of the genes conferring antibiotic resistance were found in abundant marine taxa (e.g., Pelagibac- http://aem.asm.org/ ter, Prochlorococcus, and Vibrio). Therefore, we uncovered a previously unknown diversity of genes that conferred an AR pheno- type among marine environments, which makes the ocean a global reservoir of both clinically relevant and potentially novel AR genes. he spread of antibiotic resistance (AR) is critically important sity of marine AR genes, and (iii) are these genes harbored by Tto human health. -

Tetracycline and Sulfonamide Antibiotics in Soils: Presence, Fate and Environmental Risks
processes Review Tetracycline and Sulfonamide Antibiotics in Soils: Presence, Fate and Environmental Risks Manuel Conde-Cid 1, Avelino Núñez-Delgado 2 , María José Fernández-Sanjurjo 2 , Esperanza Álvarez-Rodríguez 2, David Fernández-Calviño 1,* and Manuel Arias-Estévez 1 1 Soil Science and Agricultural Chemistry, Faculty Sciences, University Vigo, 32004 Ourense, Spain; [email protected] (M.C.-C.); [email protected] (M.A.-E.) 2 Department Soil Science and Agricultural Chemistry, Engineering Polytechnic School, University Santiago de Compostela, 27002 Lugo, Spain; [email protected] (A.N.-D.); [email protected] (M.J.F.-S.); [email protected] (E.Á.-R.) * Correspondence: [email protected] Received: 30 October 2020; Accepted: 13 November 2020; Published: 17 November 2020 Abstract: Veterinary antibiotics are widely used worldwide to treat and prevent infectious diseases, as well as (in countries where allowed) to promote growth and improve feeding efficiency of food-producing animals in livestock activities. Among the different antibiotic classes, tetracyclines and sulfonamides are two of the most used for veterinary proposals. Due to the fact that these compounds are poorly absorbed in the gut of animals, a significant proportion (up to ~90%) of them are excreted unchanged, thus reaching the environment mainly through the application of manures and slurries as fertilizers in agricultural fields. Once in the soil, antibiotics are subjected to a series of physicochemical and biological processes, which depend both on the antibiotic nature and soil characteristics. Adsorption/desorption to soil particles and degradation are the main processes that will affect the persistence, bioavailability, and environmental fate of these pollutants, thus determining their potential impacts and risks on human and ecological health. -

Sulfadimethoxine Degradation Kinetics in Manure As Affected by Initial Concentration, Moisture, and Temperature
Published online October 27, 2006 Sulfadimethoxine Degradation Kinetics in Manure as Affected by Initial Concentration, Moisture, and Temperature Q.-Q. Wang, S. A. Bradford, W. Zheng, and S. R. Yates* ABSTRACT However in Europe, a new trend has developed with in- Sulfadimethoxine is a widely used sulfonamide veterinary antibiotic creased therapeutic use of antibiotics (Alder et al., 2001). and could be a source of agricultural contamination. Therefore, infor- After the application to animals, antibiotics will even- mation is needed about its degradation kinetics in manure under aer- tually enter the environment. In fish farming, antibiotics obic conditions. Based on the analysis of first-order kinetics and the are given as feed additives, resulting in a direct release assumption that sulfadimethoxine availability for degradation in ma- of antibiotics into the aquatic environment (Thurman nure could be limiting, a new kinetic model was developed and was et al., 2002). It was estimated that |70 to 80% of drugs found to fit the degradation kinetics well. The degradation rate in administered to fish enters the environment and anti- sterile manure was found to be much lower than in nonsterile manure, biotic residues with significant antibacterial activity were indicating that biodegradation was significant. In biologically active found in the sediment of fish hatcheries (Samuelsen et al., manure, the degradation rate constant decreased with increasing ini- tial concentration of sulfadimethoxine, implying that the activity of 1992). However, the major route through which veteri- the degrading microorganisms was inhibited. Increasing moisture or nary antibiotics enter the environment is the excretion temperature was found to increase sulfadimethoxine degradation in of feces and urine from medicated animals in livestock manure. -

Sulfadimethoxine Analysis in Channel Catfish: a Model Drug Residue Monitoring Program for Aquaculture
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1993 Sulfadimethoxine Analysis in Channel Catfish: A Model Drug Residue Monitoring Program for Aquaculture. Calvin Charles Walker Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Walker, Calvin Charles, "Sulfadimethoxine Analysis in Channel Catfish: A Model Drug Residue Monitoring Program for Aquaculture." (1993). LSU Historical Dissertations and Theses. 5681. https://digitalcommons.lsu.edu/gradschool_disstheses/5681 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from theoriginal or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. -

Use of Antibiotics in Ornamental Fish Aquaculture1 Roy P
Cir 84 Use of Antibiotics in Ornamental Fish Aquaculture1 Roy P. E. Yanong2 Introduction based on their response to a protocol called gram staining. Gram-positive bacteria stain blue, and gram-negative Antibiotics are very useful additions to any fish-health bacteria stain pink. They stain differently because each manager’s toolbox, but they are only tools and not ‘magic group has a different type of outer structure known as the bullets.’ The ability of antibiotics to help eliminate a fish cell wall. This difference is important for the producer disease depends on a number of factors: 1) Does the and aquaculturist because some antibiotics work better problem actually have a bacterial component? 2) Are the against gram-positive bacteria and others work better bacteria involved sensitive to the antibiotic chosen? 3) Are against gram-negative bacteria. Most bacteria that infect the proper dosage and treatment intervals being used? 4) fish are gram-negative, including Aeromonas hydrophila, Have other contributing stresses been removed or reduced? Aeromonas salmonicida, Flavobacterium columnare (which causes columnaris), Vibrio, and Pseudomonas species. (See Antibiotics, in and of themselves, do not cure a fish. Antibi- UF/IFAS Fact Sheets FA-14 Aeromonas Infections, FA-31 otics merely control the population growth of bacteria in a Vibrio Infections of Fish and FA-11 Columnaris disease). fish long enough for its immune system to eliminate them. The major group of gram-positive bacteria that cause Before antibiotics are even considered, sources of stress disease in fish are Streptococcus. (See UF/IFAS Circular 57 such as poor water quality (including drastic temperature Streptococcal Infections in Fish.) change), nutrition, genetics, and handling or transport must A third group, the acid-fast bacteria, which includes be removed or reduced. -

The Organic Chemistry of Drug Synthesis
The Organic Chemistry of Drug Synthesis VOLUME 2 DANIEL LEDNICER Mead Johnson and Company Evansville, Indiana LESTER A. MITSCHER The University of Kansas School of Pharmacy Department of Medicinal Chemistry Lawrence, Kansas A WILEY-INTERSCIENCE PUBLICATION JOHN WILEY AND SONS, New York • Chichester • Brisbane • Toronto Copyright © 1980 by John Wiley & Sons, Inc. All rights reserved. Published simultaneously in Canada. Reproduction or translation of any part of this work beyond that permitted by Sections 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful. Requests for permission or further information should be addressed to the Permissions Department, John Wiley & Sons, Inc. Library of Congress Cataloging in Publication Data: Lednicer, Daniel, 1929- The organic chemistry of drug synthesis. "A Wiley-lnterscience publication." 1. Chemistry, Medical and pharmaceutical. 2. Drugs. 3. Chemistry, Organic. I. Mitscher, Lester A., joint author. II. Title. RS421 .L423 615M 91 76-28387 ISBN 0-471-04392-3 Printed in the United States of America 10 987654321 It is our pleasure again to dedicate a book to our helpmeets: Beryle and Betty. "Has it ever occurred to you that medicinal chemists are just like compulsive gamblers: the next compound will be the real winner." R. L. Clark at the 16th National Medicinal Chemistry Symposium, June, 1978. vii Preface The reception accorded "Organic Chemistry of Drug Synthesis11 seems to us to indicate widespread interest in the organic chemistry involved in the search for new pharmaceutical agents. We are only too aware of the fact that the book deals with a limited segment of the field; the earlier volume cannot be considered either comprehensive or completely up to date. -

Sulfonamides and Sulfonamide Combinations*
Sulfonamides and Sulfonamide Combinations* Overview Due to low cost and relative efficacy against many common bacterial infections, sulfonamides and sulfonamide combinations with diaminopyrimidines are some of the most common antibacterial agents utilized in veterinary medicine. The sulfonamides are derived from sulfanilamide. These chemicals are structural analogues of ρ-aminobenzoic acid (PABA). All sulfonamides are characterized by the same chemical nucleus. Functional groups are added to the amino group or substitutions made on the amino group to facilitate varying chemical, physical and pharmacologic properties and antibacterial spectra. Most sulfonamides are too alkaline for routine parenteral use. Therefore the drug is most commonly administered orally except in life threatening systemic infections. However, sulfonamide preparations can be administered orally, intramuscularly, intravenously, intraperitoneally, intrauterally and topically. Sulfonamides are effective against Gram-positive and Gram-negative bacteria. Some protozoa, such as coccidians, Toxoplasma species and plasmodia, are generally sensitive. Chlamydia, Nocardia and Actinomyces species are also sensitive. Veterinary diseases commonly treated by sulfonamides are actinobacillosis, coccidioidosis, mastitis, metritis, colibacillosis, pododermatitis, polyarthritis, respiratory infections and toxo- plasmosis. Strains of rickettsiae, Pseudomonas, Klebsiella, Proteus, Clostridium and Leptospira species are often highly resistant. Sulfonamides are bacteriostatic antimicrobials -

United States Patent (19) 11) Patent Number: 5,001,115 Sloan (45) Date of Patent: Mar
United States Patent (19) 11) Patent Number: 5,001,115 Sloan (45) Date of Patent: Mar. 19, 1991 54 PRODRUGS OF BIOLOGICALLY ACTIVE Primary Examiner-Mukund J. Shah HYDROXYAROMATIC COMPOUNDS Assistant Examiner-E. L. Ward Attorney, Agent, or Firm-Kerkam, Stowell, Kondracki (75 Inventor: Kenneth B. Sloan, Gainesville, Fla. & Clarke 73 Assignee: University of Florida, Gainesville, Fla, (57) ABSTRACT (21) Appl. No.: 352,919 Prodrugs of bio-active hydroxyaromatic drugs having 22 Filed: May 17, 1989 the structural formula: A pharmaceutically acceptable prodrug of a biologi (51] Int. Cl. ............... A61K 31/70; A61K 31/595; cally active, therapeutically effective hydroxyaromatic A61K 31/535; A61K 31/255 52 U.S. C. ..................................... 514/34: 514/289; drug, said prodrug being selected from the group con 514/169; 514/373; 514/222.8; 514/328; sisting of, (A) compounds having the structural for 514/360; 514/603; 514/417; 514/425; 514/518; mula: 546/44; 54.6/176; 546/75; 544/2; 536/64; 548/123; 548/209; 548/256; 548/417 DRUG-O-CR'R''-2) 58 Field of Search ............... 514/169,289, 373, 417, 514/425, 222.8, 328,360, 518, 603, 34; 536/64; wherein: 564/82, 155; 552/626; 546/44, 176, 75; 544/2; DRUG -O- is the hydroxyaromatic O-dehydro 548/123, 209, 256, 477, 595 residue of said drug; 56 References Cited R" and R' may be the same or different and may be H, PUBLICATIONS alkyl, aryl or electron withdrawing groups; 2 is a displaceable leaving group; and Katritzky, et al. J. Chem. Soc. -

On the Extraction of Antibiotics from Shrimps Prior to Chromatographic Analysis
separations Review On the Extraction of Antibiotics from Shrimps Prior to Chromatographic Analysis Victoria Samanidou *, Dimitrios Bitas, Stamatia Charitonos and Ioannis Papadoyannis Laboratory of Analytical Chemistry, University of Thessaloniki, GR 54124 Thessaloniki, Greece; [email protected] (D.B.); [email protected] (S.C.); [email protected] (I.P.) * Correspondence: [email protected]; Tel.: +30-2310-997698; Fax: +30-2310-997719 Academic Editor: Frank L. Dorman Received: 2 February 2016; Accepted: 26 February 2016; Published: 4 March 2016 Abstract: The widespread use of antibiotics in veterinary practice and aquaculture has led to the increase of antimicrobial resistance in food-borne pathogens that may be transferred to humans. Global concern is reflected in the regulations from different agencies that have set maximum permitted residue limits on antibiotics in different food matrices of animal origin. Sensitive and selective methods are required to monitor residue levels in aquaculture species for routine regulatory analysis. Since sample preparation is the most important step, several extraction methods have been developed. In this review, we aim to summarize the trends in extraction of several antibiotics classes from shrimps and give a comparison of performance characteristics in the different approaches. Keywords: sample preparation; extraction; aquaculture; shrimps; chromatography; antibiotics 1. Introduction According to FAO (CWP Handbook of Fishery Statistical Standards, Section J: AQUACULTURE), “aquaculture is the farming of aquatic organisms: fish, mollusks, crustaceans, aquatic plants, crocodiles, alligators, turtles, and amphibians. Farming implies some form of intervention in the rearing process to enhance production, such as regular stocking, feeding, protection from predators, etc.”[1]. Since 1960, aquaculture practice and production has increased as a result of the improved conditions in the aquaculture facilities. -

Swedres-Svarm 2004
SVARM2004 Swedish Veterinary Antimicrobial Resistance Monitoring Preface .............................................................................................4 Summary ..........................................................................................6 Sammanfattning ...............................................................................7 Use of antimicrobials .........................................................................8 Resistance in zoonotic bacteria ......................................................12 Salmonella ..........................................................................................................12 Campylobacter ...................................................................................................15 Resistance in indicator bacteria ......................................................17 Escherichia coli ...................................................................................................17 Enterococcus .....................................................................................................20 Resistance in animal pathogens ......................................................28 Pig ......................................................................................................................28 Cattle ..................................................................................................................29 Horse ..................................................................................................................30