Wo 2010/059253 A2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
(12) United States Patent (10) Patent No.: US 8,993,581 B2 Perrine Et Al
US00899.3581B2 (12) United States Patent (10) Patent No.: US 8,993,581 B2 Perrine et al. (45) Date of Patent: Mar. 31, 2015 (54) METHODS FOR TREATINGVIRAL (58) Field of Classification Search DSORDERS CPC ... A61K 31/00; A61K 31/166; A61K 31/185: A61K 31/233; A61K 31/522: A61K 38/12: (71) Applicant: Trustees of Boston University, Boston, A61K 38/15: A61K 45/06 MA (US) USPC ........... 514/263.38, 21.1, 557, 565, 575, 617; 424/2011 (72) Inventors: Susan Perrine, Weston, MA (US); Douglas Faller, Weston, MA (US) See application file for complete search history. (73) Assignee: Trustees of Boston University, Boston, (56) References Cited MA (US) U.S. PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this 3,471,513 A 10, 1969 Chinn et al. patent is extended or adjusted under 35 3,904,612 A 9/1975 Nagasawa et al. U.S.C. 154(b) by 0 days. (Continued) (21) Appl. No.: 13/915,092 FOREIGN PATENT DOCUMENTS (22) Filed: Jun. 11, 2013 CA 1209037 A 8, 1986 CA 2303268 A1 4f1995 (65) Prior Publication Data (Continued) US 2014/OO45774 A1 Feb. 13, 2014 OTHER PUBLICATIONS Related U.S. Application Data (63) Continuation of application No. 12/890,042, filed on PCT/US 10/59584 Search Report and Written Opinion mailed Feb. Sep. 24, 2010, now abandoned. 11, 2011. (Continued) (60) Provisional application No. 61/245,529, filed on Sep. 24, 2009, provisional application No. 61/295,663, filed on Jan. 15, 2010. Primary Examiner — Savitha Rao (74) Attorney, Agent, or Firm — Nixon Peabody LLP (51) Int. -

Newer Trends in the Management of Genital Herpes
Review NNewerewer trendstrends inin thethe mmanagementanagement ofof genitalgenital herpesherpes Article AAmiyamiya KKumarumar NNath,ath, DDevinderevinder MohanMohan ThappaThappa Department of Dermatology ABSTRACT and STD, Jawaharlal Institute of Postgraduate Medical Management of genital herpes is complex. Apart from using the standard antivirals, an Education and Research (JIPMER), Pondicherry - 605 ideal management protocol also needs to address various aspects of the disease, including 006, India the psychological morbidity. Oral acyclovir, valacyclovir or famciclovir are recommended for routine use. Long-term suppressive therapy is effective in reducing the number of AAddressddress forfor ccorrespondence:orrespondence: recurrences and the risk of transmission to others. Severe or disseminated disease may Dr. Devinder Mohan Thappa, require intravenous therapy. Resistant cases are managed with foscarnet or cidofovir. Genital Department of Dermatology herpes in human immunodeÞ ciency virus-infected individuals usually needs a longer duration and STD, JIPMER, of antiviral therapy along with continuation of highly active anti retroviral therapy (HAART). Pondicherry - 605 006, India. E-mail: [email protected] Genital herpes in late pregnancy increases the risk of neonatal herpes. Antiviral therapy and/or cesarean delivery are indicated depending on the clinical circumstance. Acyclovir appears to be safe in pregnancy. But, there is limited data regarding the use of valacyclovir and famciclovir in pregnancy. Neonatal herpes requires a higher dose of acyclovir given intravenously for a longer duration. Management of the sex partner, counseling and prevention advice are equally important in appropriate management of genital herpes. Vaccines till date have been marginally effective. Helicase–primase inhibitors, needle-free mucosal vaccine and a new microbicide product named VivaGel may become promising treatment options in the future. -
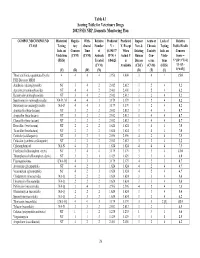
2002 FSIS National Residue Program, Section 4
Table 4.1 Scoring Table for Veterinary Drugs 2002 FSIS NRP, Domestic Monitoring Plan COMPOUND/COMPOUND Historical Regula- With- Relative Predicted Predicted Impact Acute or Lack of Relative CLASS Testing tory drawal Number V = V, Except New & Chronic Testing Public Health Info. on Concern Time of (0.19437* When Existing Toxicity Info. on Concern Violations (CVM) (CVM) Animals R*N) + Actual V Human Con- Viola- Score = (FSIS) Treated 0.84625 is Disease cerns tions V*[(D+3*T)/4] (CVM) Available (CDC) (CVM) (FSIS) *{1+[(L- (V) (R) (W) (N) (D) (T) (L) 1)*0.05]} Those antibiotics quantitated by the 4 4 4 4 3.956 4.000 3 4 1 15.0 FSIS Bioassay MRM Amikacin (aminoglycoside) NT 3 4 2 2.012 2.012 3 2 4 5.2 Apramycin (aminoglycoside) NT 4 4 2 2.401 2.401 3 2 4 6.2 Kanamycin (aminoglycoside) NT 3 4 2 2.012 2.012 3 2 4 5.2 Spectinomycin (aminoglycoside) NA-D, M 4 4 3 3.179 3.179 3 2 4 8.2 Streptomycin (aminoglycoside) NA-D 4 4 3 3.179 3.179 3 2 4 8.2 Amoxicillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Ampicillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Cloxacillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Hetacillin (beta-lactam) NT 2 2 2 1.624 1.624 3 4 4 7.0 Ticarcillin (beta-lactam) NT 2 2 2 1.624 1.624 3 4 4 7.0 Ceftiofur (cefalosporin) NT 3 2 3 2.596 2.596 4 2 4 7.5 Cefazolin (synthetic cefalosporin) NT 3 2 2 2.012 2.012 3 2 4 5.2 Chloramphenicol NA-N 4 2 1 1.624 1.624 4 4 4 7.5 Florfenicol (chloramphen. -

The Effects of Atopy and Asthma on in Vivo Human Nasal Responses to Toll
The effects of atopy and asthma on in vivo human nasal responses to Toll-like receptor agonists Dr Akhilesh Jha A thesis submitted for the degree of Doctor of Philosophy (PhD) Centre for Respiratory Infection National Heart and Lung Institute (NHLI), St Mary’s Campus Imperial College London, Norfolk Place, London W2 1PG July 2018 Abstract Acute respiratory viral infections cause significant morbidity and mortality, especially in vulnerable individuals, and it is important to study viral pathogenesis and the host immune response in humans. Toll-like receptors (TLRs) play a critical role in the detection of viral nucleic acids, and airway TLR receptors respond to nucleic acid patterns in the RNA viruses that cause respiratory infections. However, a reliable method of measuring mucosal innate immune responses to viral infections is lacking. TLR3 agonists (poly(I:C) and poly-ICLC) and the combined TLR7/8 agonist (resiquimod, R848) are synthetic analogues of double stranded RNA (dsRNA) and single stranded RNA (ssRNA) respectively. Nasal challenge with these TLR agonists was carried out, and serial sampling using nasosorption and nasal curettage was performed. Mucosal immune responses were measured and the effect of different host factors (e.g. asthma) on these responses was studied. Poly(I:C) and poly-ICLC were well tolerated but failed to induce significant and reliable nasal mucosal innate immune responses. R848 at a higher dose (10 µg/100 µL per nostril) induced significant mucosal interferon and cytokine responses but caused mild to moderate flu-like symptoms in three out of nine volunteers. A lower dose of R848 (0.02 µg/kg/100 µL, mean dose 1.5 µg/100 µL) was subsequently utilised in three groups of volunteers: healthy non-atopic (n=12), allergic rhinitis (n=12) and allergic asthma (n=11). -

Inclusion and Exclusion Criteria for Each Key Question
Supplemental Table 1: Inclusion and exclusion criteria for each key question Chronic HBV infection in adults ≥ 18 year old (detectable HBsAg in serum for >6 months) Definition of disease Q1 Q2 Q3 Q4 Q5 Q6 Q7 HBV HBV infection with infection and persistent compensated Immunoactive Immunotolerant Seroconverted HBeAg HBV mono-infected viral load cirrhosis with Population chronic HBV chronic HBV from HBeAg to negative population under low level infection infection anti-HBe entecavir or viremia tenofovir (<2000 treatment IU/ml) Adding 2nd Stopped antiviral therapy antiviral drug Interventions and Entecavir compared Antiviral Antiviral therapy compared to continued compared to comparisons to tenofovir therapy therapy continued monotherapy Q1-2: Clinical outcomes: Cirrhosis, decompensated liver disease, HCC and death Intermediate outcomes (if evidence on clinical outcomes is limited or unavailable): HBsAg loss, HBeAg seroconversion and Outcomes HBeAg loss Q3-4: Cirrhosis, decompensated liver disease, HCC, relapse (viral and clinical) and HBsAg loss Q5: Renal function, hypophosphatemia and bone density Q6: Resistance, flare/decompensation and HBeAg loss Q7: Clinical outcomes: Cirrhosis, decompensated liver disease, HCC and death Study design RCT and controlled observational studies Acute HBV infection, children and pregnant women, HIV (+), HCV (+) or HDV (+) persons or other special populations Exclusions such as hemodialysis, transplant, and treatment failure populations. Co treatment with steroids and uncontrolled studies. Supplemental Table 2: Detailed Search Strategy: Ovid Database(s): Embase 1988 to 2014 Week 37, Ovid MEDLINE(R) In-Process & Other Non- Indexed Citations and Ovid MEDLINE(R) 1946 to Present, EBM Reviews - Cochrane Central Register of Controlled Trials August 2014, EBM Reviews - Cochrane Database of Systematic Reviews 2005 to July 2014 Search Strategy: # Searches Results 1 exp Hepatitis B/dt 26410 ("hepatitis B" or "serum hepatitis" or "hippie hepatitis" or "injection hepatitis" or 2 178548 "hepatitis type B").mp. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Bioinformatics Review of the Role of HSV-1 in Alzheimer's Disease
Advances in Alzheimer’s Disease, 2020, 9, 57-75 https://www.scirp.org/journal/aad ISSN Online: 2169-2467 ISSN Print: 2169-2459 Bioinformatics Review of the Role of HSV-1 in Alzheimer’s Disease Brian T. Reiss1, Meade C. Eggleston1, Arah C. Godbole1, Julia A. Marut1, Cecelia M. McCann1, Jessica A. Cottrell1, Tinchun Chu1, Sulie L. Chang1,2* 1Department of Biological Sciences, Seton Hall University, South Orange, NJ, USA 2Institute of Neuroimmune Pharmacology, Seton Hall University, South Orange, NJ, USA How to cite this paper: Reiss, B.T., Eg- Abstract gleston, M.C., Godbole, A.C., Marut, J.A., McCann, C.M., Cottrell, J.A., Chu, T.C. and Alzheimer’s disease (AD) is a neurodegenerative disease characterized by the Chang, S.L. (2020) Bioinformatics Review progressive loss of cognitive functions in affected individuals. Brain tissue of the Role of HSV-1 in Alzheimer’s Dis- pathology is associated with the formation of senile plaques which result from ease. Advances in Alzheimer’s Disease, 9, 57-75. the over-production of amyloid β (Aβ), due to the cleavage of a membrane https://doi.org/10.4236/aad.2020.93005 bound glycoprotein. It is unclear what causes AD and its associated patholo- gies, but age and genetic predisposition play an import role in the likelihood Received: June 11, 2020 Accepted: September 6, 2020 of disease development. Studies have shown that the reactivation of latent Published: September 9, 2020 herpes simplex virus 1 (HSV-1) infection can lead to the neuropathy of acute herpes simplex encephalitis (HSE), which causes similar symptoms to AD. -

A Screening-Based Approach to Circumvent Tumor Microenvironment
JBXXXX10.1177/1087057113501081Journal of Biomolecular ScreeningSingh et al. 501081research-article2013 Original Research Journal of Biomolecular Screening 2014, Vol 19(1) 158 –167 A Screening-Based Approach to © 2013 Society for Laboratory Automation and Screening DOI: 10.1177/1087057113501081 Circumvent Tumor Microenvironment- jbx.sagepub.com Driven Intrinsic Resistance to BCR-ABL+ Inhibitors in Ph+ Acute Lymphoblastic Leukemia Harpreet Singh1,2, Anang A. Shelat3, Amandeep Singh4, Nidal Boulos1, Richard T. Williams1,2*, and R. Kiplin Guy2,3 Abstract Signaling by the BCR-ABL fusion kinase drives Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) and chronic myelogenous leukemia (CML). Despite their clinical activity in many patients with CML, the BCR-ABL kinase inhibitors (BCR-ABL-KIs) imatinib, dasatinib, and nilotinib provide only transient leukemia reduction in patients with Ph+ ALL. While host-derived growth factors in the leukemia microenvironment have been invoked to explain this drug resistance, their relative contribution remains uncertain. Using genetically defined murine Ph+ ALL cells, we identified interleukin 7 (IL-7) as the dominant host factor that attenuates response to BCR-ABL-KIs. To identify potential combination drugs that could overcome this IL-7–dependent BCR-ABL-KI–resistant phenotype, we screened a small-molecule library including Food and Drug Administration–approved drugs. Among the validated hits, the well-tolerated antimalarial drug dihydroartemisinin (DHA) displayed potent activity in vitro and modest in vivo monotherapy activity against engineered murine BCR-ABL-KI–resistant Ph+ ALL. Strikingly, cotreatment with DHA and dasatinib in vivo strongly reduced primary leukemia burden and improved long-term survival in a murine model that faithfully captures the BCR-ABL-KI–resistant phenotype of human Ph+ ALL. -

Tepzz¥Z8 4A T
(19) TZZ¥Z_ _ T (11) EP 3 081 214 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 19.10.2016 Bulletin 2016/42 A61K 31/4178 (2006.01) A61K 31/422 (2006.01) A61P 25/28 (2006.01) (21) Application number: 16000020.4 (22) Date of filing: 30.08.2004 (84) Designated Contracting States: • Yuan, Junying, AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Newton, HU IE IT LI LU MC NL PL PT RO SE SI SK TR MA 02468 (US) • Jagtap, Prakash, (30) Priority: 29.08.2003 US 498882 P North Andover, MA 01845 (US) (62) Document number(s) of the earlier application(s) in • Degterev, Alexei, accordance with Art. 76 EPC: Brookline, 10011481.8 / 2 384 753 MA 02445 (US) 04821344.1 / 1 663 184 (74) Representative: Bösl, Raphael Konrad (71) Applicants: Isenbruck Bösl Hörschler LLP • THE BRIGHAM AND WOMEN’S HOSPITAL, INC. Patentanwälte Boston, MA 02115 (US) Prinzregentenstraße 68 • President and Fellows of Harvard College 81675 München (DE) Cambridge, MA 02138 (US) Remarks: (72) Inventors: •This application was filed on 05-01-2016 as a • Cuny, Gregory D., divisional application to the application mentioned Houston, under INID code 62. TX 77004 (US) •Claims filed after the date of filing of the application (Rule 68(4) EPC). (54) INHIBITORS OF CELLULAR NECROSIS (57) The present invention relates to compounds and pharmaceutical preparations and their use in therapy for preventing or treating trauma, ischemia, stroke and degenerative diseases associated with cell death. Methods and compositions of the invention are particularly useful for treating neurological disorders associated with cellular necrosis. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

2019-6654 Final Report of an Audit Carried out In
Ref. Ares(2019)7826731 - 19/12/2019 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Health and food audits and analysis DG(SANTE) 2019-6654 FINAL REPORT OF AN AUDIT CARRIED OUT IN BELARUS FROM 13 TO 24 MAY 2019 IN ORDER TO EVALUATE THE CONTROL OF RESIDUES AND CONTAMINANTS IN LIVE ANIMALS AND ANIMAL PRODUCTS INCLUDING CONTROLS ON VETERINARY MEDICINAL PRODUCTS Executive Summary This report describes the outcome of an audit carried out in Belarus from 13 to 24 May 2019 as part of the European Commission’s Directorate-General for Health and Food Safety planned work programme. The objective of the audit was to evaluate the effectiveness of official controls on residues and contaminants in live animals and animal products eligible for export to the European Union (EU). The audit assessed the implementation of the residue monitoring plan and also covered the authorisation, distribution and use of veterinary medicinal products, given that these areas have an impact on the monitoring of residues. Attention was also paid to examining the implementation of corrective actions indicated in response to specific recommendations made in the report of the previous residues audit to Belarus. The planning of the residue monitoring largely follows the principles of Directive 96/23/EC and covers for the most part an appropriate range of substances. The plan is nevertheless weakened by the fact that action levels for several substances across all commodities (including those for which listing has been requested) are not aligned with EU maximum residue limits, thus the plan would not be sufficient to demonstrate that commodities eligible for export to the EU would comply with such limits where they are lower that national limits.