Disrupted Functional Connectivity Between Sub-Regions in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insular Volume Reductions in Patients with Major Depressive Disorder
Insular volume reductions in patients with major depressive disorder Item Type Article Authors Mutschler, Isabella; Hänggi, Jürgen; Frei, Manuela; Lieb, Roselind; grosse Holforth, Martin; Seifritz, Erich; Spinelli, Simona Citation Mutschler, I., Hänggi, J., Frei, M., Lieb, R., grosse Holforth, M., Seifritz, E., & Spinelli, S. (2019). Insular volume reductions in patients with major depressive disorder. Neurology, Psychiatry and Brain Research, 33, 39–47. doi:10.1016/j.npbr.2019.06.002 Eprint version Post-print DOI 10.1016/j.npbr.2019.06.002 Publisher Elsevier BV Journal Neurology Psychiatry and Brain Research Rights NOTICE: this is the author’s version of a work that was accepted for publication in Neurology Psychiatry and Brain Research. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality control mechanisms may not be reflected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was subsequently published in Neurology Psychiatry and Brain Research, [[Volume], [Issue], (2019-06-22)] DOI: 10.1016/ j.npbr.2019.06.002 . © 2019. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http:// creativecommons.org/licenses/by-nc-nd/4.0/ Download date 23/09/2021 13:26:26 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10754/656271 Neurology, Psychiatry and Brain Research 33 (2019) 39–47 Contents lists available at ScienceDirect Neurology, -

Cortical Abnormalities in Bipolar Disorder: an MRI Analysis of 6503 Individuals from the ENIGMA Bipolar Disorder Working Group
OPEN Molecular Psychiatry (2018) 23, 932–942 www.nature.com/mp ORIGINAL ARTICLE Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group DP Hibar1,2, LT Westlye3,4,5, NT Doan3,4, N Jahanshad1, JW Cheung1, CRK Ching1,6, A Versace7, AC Bilderbeck8, A Uhlmann9,10, B Mwangi11, B Krämer12, B Overs13, CB Hartberg3, C Abé14, D Dima15,16, D Grotegerd17, E Sprooten18, E Bøen19, E Jimenez20, FM Howells9, G Delvecchio21, H Temmingh9, J Starke9, JRC Almeida22, JM Goikolea20, J Houenou23,24, LM Beard25, L Rauer12, L Abramovic26, M Bonnin20, MF Ponteduro16, M Keil27, MM Rive28,NYao29,30, N Yalin31, P Najt32, PG Rosa33,34, R Redlich17, S Trost27, S Hagenaars35, SC Fears36,37, S Alonso-Lana38,39, TGM van Erp40, T Nickson35, TM Chaim-Avancini33,34, TB Meier41,42, T Elvsåshagen3,43, UK Haukvik3,44, WH Lee18, AH Schene45,46, AJ Lloyd47, AH Young31, A Nugent48, AM Dale49,50, A Pfennig51, AM McIntosh35, B Lafer33, BT Baune52, CJ Ekman14, CA Zarate48, CE Bearden53,54, C Henry23,55, C Simhandl56, C McDonald32, C Bourne8,57, DJ Stein9,10, DH Wolf25, DM Cannon32, DC Glahn29,30, DJ Veltman58, E Pomarol-Clotet38,39, E Vieta20, EJ Canales-Rodriguez38,39, FG Nery33,59, FLS Duran33,34, GF Busatto33,34, G Roberts60, GD Pearlson29,30, GM Goodwin8, H Kugel61, HC Whalley35, HG Ruhe8,28,62, JC Soares11, JM Fullerton13,63, JK Rybakowski64, J Savitz42,65, KT Chaim66,67, M Fatjó-Vilas38,39, MG Soeiro-de-Souza33, MP Boks26, MV Zanetti33,34, MCG Otaduy66,67, MS Schaufelberger33,34, M Alda68, M Ingvar14,69, -

Neural Correlates Underlying Change in State Self-Esteem Hiroaki Kawamichi 1,2,3, Sho K
www.nature.com/scientificreports OPEN Neural correlates underlying change in state self-esteem Hiroaki Kawamichi 1,2,3, Sho K. Sugawara2,4,5, Yuki H. Hamano2,5,6, Ryo Kitada 2,7, Eri Nakagawa2, Takanori Kochiyama8 & Norihiro Sadato 2,5 Received: 21 July 2017 State self-esteem, the momentary feeling of self-worth, functions as a sociometer involved in Accepted: 11 January 2018 maintenance of interpersonal relations. How others’ appraisal is subjectively interpreted to change Published: xx xx xxxx state self-esteem is unknown, and the neural underpinnings of this process remain to be elucidated. We hypothesized that changes in state self-esteem are represented by the mentalizing network, which is modulated by interactions with regions involved in the subjective interpretation of others’ appraisal. To test this hypothesis, we conducted task-based and resting-state fMRI. Participants were repeatedly presented with their reputations, and then rated their pleasantness and reported their state self- esteem. To evaluate the individual sensitivity of the change in state self-esteem based on pleasantness (i.e., the subjective interpretation of reputation), we calculated evaluation sensitivity as the rate of change in state self-esteem per unit pleasantness. Evaluation sensitivity varied across participants, and was positively correlated with precuneus activity evoked by reputation rating. Resting-state fMRI revealed that evaluation sensitivity was positively correlated with functional connectivity of the precuneus with areas activated by negative reputation, but negatively correlated with areas activated by positive reputation. Thus, the precuneus, as the part of the mentalizing system, serves as a gateway for translating the subjective interpretation of reputation into state self-esteem. -

Supplementary Tables
Supplementary Tables: ROI Atlas Significant table grey matter Test ROI # Brainetome area beta volume EG pre vs post IT 8 'superior frontal gyrus, part 4 (dorsolateral area 6), right', 0.773 17388 11 'superior frontal gyrus, part 6 (medial area 9), left', 0.793 18630 12 'superior frontal gyrus, part 6 (medial area 9), right', 0.806 24543 17 'middle frontal gyrus, part 2 (inferior frontal junction), left', 0.819 22140 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1.3 10665 67 'paracentral lobule, part 2 (area 4 lower limb), left', 0.86 13662 EG pre vs post ET 20 'middle frontal gyrus, part 3 (area 46), right', 0.934 28188 21 'middle frontal gyrus, part 4 (ventral area 9/46 ), left' 0.812 27864 31 'inferior frontal gyrus, part 2 (inferior frontal sulcus), left', 0.864 11124 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1 10665 50 'orbital gyrus, part 5 (area 13), right', -1.7 22626 67 'paracentral lobule, part 2 (area 4 lower limb), left', 1.1 13662 180 'cingulate gyrus, part 3 (pregenual area 32), right', 0.9 10665 261 'Cerebellar lobule VIIb, vermis', -1.5 729 IG pre vs post IT 16 middle frontal gyrus, part 1 (dorsal area 9/46), right', -0.8 27567 24 'middle frontal gyrus, part 5 (ventrolateral area 8), right', -0.8 22437 40 'inferior frontal gyrus, part 6 (ventral area 44), right', -0.9 8262 54 'precentral gyrus, part 1 (area 4 head and face), right', -0.9 14175 64 'precentral gyrus, part 2 (caudal dorsolateral area 6), left', -1.3 18819 81 'middle temporal gyrus, part 1 (caudal area 21), left', -1.4 14472 -

Seed MNI Coordinates Lobe
MNI Coordinates Seed Lobe (Hemisphere) Region BAa X Y Z FP1 -18 62 0 Frontal Lobe (L) Medial Frontal Gyrus 10 FPz 4 62 0 Frontal Lobe (R) Medial Frontal Gyrus 10 FP2 24 60 0 Frontal Lobe (R) Superior Frontal Gyrus 10 AF7 -38 50 0 Frontal Lobe (L) Middle Frontal Gyrus 10 AF3 -30 50 24 Frontal Lobe (L) Superior Frontal Gyrus 9 AFz 4 58 30 Frontal Lobe (R) Medial Frontal Gyrus 9 AF4 36 48 20 Frontal Lobe (R) Middle Frontal Gyrus 10 AF8 42 46 -4 Frontal Lobe (R) Inferior Frontal Gyrus 10 F7 -48 26 -4 Frontal Lobe (L) Inferior Frontal Gyrus 47 F5 -48 28 18 Frontal Lobe (L) Inferior Frontal Gyrus 45 F3 -38 28 38 Frontal Lobe (L) Precentral Gyrus 9 F1 -20 30 50 Frontal Lobe (L) Superior Frontal Gyrus 8 Fz 2 32 54 Frontal Lobe (L) Superior Frontal Gyrus 8 F2 26 32 48 Frontal Lobe (R) Superior Frontal Gyrus 8 F4 42 30 34 Frontal Lobe (R) Precentral Gyrus 9 F6 50 28 14 Frontal Lobe (R) Middle Frontal Gyrus 46 F8 48 24 -8 Frontal Lobe (R) Inferior Frontal Gyrus 47 FT9 -50 -6 -36 Temporal Lobe (L) Inferior Temporal Gyrus 20 FT7 -54 2 -8 Temporal Lobe (L) Superior Temporal Gyrus 22 FC5 -56 4 22 Frontal Lobe (L) Precentral Gyrus 6 FC3 -44 6 48 Frontal Lobe (L) Middle Frontal Gyrus 6 FC1 -22 6 64 Frontal Lobe (L) Middle Frontal Gyrus 6 FCz 4 6 66 Frontal Lobe (R) Medial Frontal Gyrus 6 FC2 28 8 60 Frontal Lobe (R) Sub-Gyral 6 FC4 48 8 42 Frontal Lobe (R) Middle Frontal Gyrus 6 FC6 58 6 16 Frontal Lobe (R) Inferior Frontal Gyrus 44 FT8 54 2 -12 Temporal Lobe (R) Superior Temporal Gyrus 38 FT10 50 -6 -38 Temporal Lobe (R) Inferior Temporal Gyrus 20 T7/T3 -

Functional Connectivity of the Precuneus in Unmedicated Patients with Depression
Biological Psychiatry: CNNI Archival Report Functional Connectivity of the Precuneus in Unmedicated Patients With Depression Wei Cheng, Edmund T. Rolls, Jiang Qiu, Deyu Yang, Hongtao Ruan, Dongtao Wei, Libo Zhao, Jie Meng, Peng Xie, and Jianfeng Feng ABSTRACT BACKGROUND: The precuneus has connectivity with brain systems implicated in depression. METHODS: We performed the first fully voxel-level resting-state functional connectivity (FC) neuroimaging analysis of depression of the precuneus, with 282 patients with major depressive disorder and 254 control subjects. RESULTS: In 125 unmedicated patients, voxels in the precuneus had significantly increased FC with the lateral orbitofrontal cortex, a region implicated in nonreward that is thereby implicated in depression. FC was also increased in depression between the precuneus and the dorsolateral prefrontal cortex, temporal cortex, and angular and supramarginal areas. In patients receiving medication, the FC between the lateral orbitofrontal cortex and precuneus was decreased back toward that in the control subjects. In the 254 control subjects, parcellation revealed superior anterior, superior posterior, and inferior subdivisions, with the inferior subdivision having high connectivity with the posterior cingulate cortex, parahippocampal gyrus, angular gyrus, and prefrontal cortex. It was the ventral subdivision of the precuneus that had increased connectivity in depression with the lateral orbitofrontal cortex and adjoining inferior frontal gyrus. CONCLUSIONS: The findings support the theory that the system in the lateral orbitofrontal cortex implicated in the response to nonreceipt of expected rewards has increased effects on areas in which the self is represented, such as the precuneus. This may result in low self-esteem in depression. The increased connectivity of the precuneus with the prefrontal cortex short-term memory system may contribute to the rumination about low self-esteem in depression. -

Supplementary Figure 1 A
100 r = 0.82 100 r = 0.87 100 r = 0.93 p = 0.002 p = 0.001 p < 0.001 90 90 90 80 80 80 VVIQ_1 VVIQ_2 VVIQ_1 70 70 70 60 60 60 55 60 65 70 75 80 85 90 95 100 55 60 65 70 75 80 85 90 95 100 55 60 65 70 75 80 85 90 95 100 VVIQ_2 VVIQ_3 VVIQ_3 100 100 100 r = 0.78 r = 0,85 R = 0.90 90 p = 0.005 90 p = 0.001 90 p < 0.001 80 80 80 70 70 70 VVIQ_S1 VVIQ_S1 60 60 VVIQ_S2 60 50 50 50 40 40 40 40 50 60 70 80 90 100 40 50 60 70 80 90 100 40 50 60 70 80 90 100 VVIQ_S2 VVIQ_S3 VVIQ_S3 Supplementary Figure 1 A. SES (Sensory Component) piercing durchstoßen hot heiss hammering hämmernd stinging stechend glowing glühend throbbing pochend tearing reißend burning brennend knocking klopfend cutting* * schneidend* 0 1 2 3 4 5 B. SES (Affective Component) disabling lähmend unbearable unerträglich terrible furchtbar torturing marternd enervating entnervend heavy schwer nasty scheusslich awful schauderhaft miserable elend murdering mörderisch violent heftig exhaustive erschöpfend cruel grausam excrutiating quälend 0 1 2 3 4 5 Supplementary Figure 2 Supplemental Table 1: Group level (N=10) brain activation during pain Imagination (A.), pain memory (B.) and pain imagination vs. pain memory (C.). A. Pain Imagination Cluster T-contrast Brain area BA x,y,z size t value Activations Right Hemisphere Frontal lobe 56,-2,16 5 4.47 Cerebellum anterior lobe, 2,-46,-6 22 6.48 Vermis 4-5 19 Cerebellum posterior lobe,inferior semi-lunar lobe 12,-72,-50 12 6.11 Activations Left Hemisphere Precentral gyrus (face) -54,-8,38 14 4.65 Postcentral gyrus (tooth) -60,-18,26 30 5.01 -
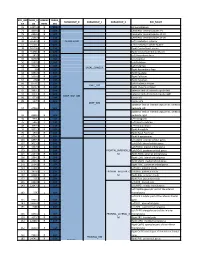
Core-Example1.Pdf
ROI_IND NUM_V HEMISP TISSUE_ SUBGROUP_0 SUBGROUP_1 SUBGROUP_2 ROI_NAME EX OX HERE SEG 95 12871.8 B WM corpus callosum 71 4899.8 B GM Cerebellar Vermal Lobules I-V 73 2858.8 B GM Cerebellar Vermal Lobules VIII-X 72 2266.9 B GM Cerebellar Vermal Lobules VI-VII 39 54582.6 L GM CEREBELLUM Left Cerebellum Exterior 41 15500.7 L WM Left Cerebellum White Matter 38 54379.4 R GM Right Cerebellum Exterior 40 15458.7 R WM Right Cerebellum White Matter 30 585.9 L GM Left Accumbens Area 37 3578.9 L GM Left Caudate 56 1597.6 L GM Left Pallidum 58 4942.3 L GM Left Putamen BASAL_GANGLIA 23 526 R GM Right Accumbens Area 36 3651.5 R GM Right Caudate 55 1638.8 R GM Right Pallidum 57 4726 R GM Right Putamen 60 8574.1 L GM Left Thalamus Proper DEEP_GM 59 8256.3 R GM Right Thalamus Proper 92 2887.7 L WM anterior limb of internal capsule left 91 3393.3 R WM anterior limb of internal capsule right DEEP_WM_GM 90 673.6 L WM fornix left 89 517.5 R WM fornix right DEEP_WM posterior limb of internal capsule inc. cerebral 94 2416.3 L WM peduncle left posterior limb of internal capsule inc. cerebral 93 2480.5 R WM peduncle right 32 993.7 L GM Left Amygdala 75 586.5 L GM Left Basal Forebrain 48 3597.7 L GM Left Hippocampus 31 1021.3 R GM Right Amygdala 76 593.1 R GM Right Basal Forebrain 47 3704.7 R GM Right Hippocampus 105 1897.7 L GM Left AOrG anterior orbital gyrus 137 3015.9 L GM Left LOrG lateral orbital gyrus 147 4637.3 L GM Left MOrG medial orbital gyrus 179 2915.7 L GM FRONTAL_INFERIOR_G Left POrG posterior orbital gyrus 104 2244.9 R GM M Right AOrG anterior orbital -

Stroke - Overview
Stroke - Overview Dr Natasha Gerbis Neurology Registrar Royal North Shore Hospital Acknowledgement Thank you Dr Jason Gu Overview ● Anatomy ● Pathophysiology ○ The CNS: an overview ○ Ischaemic stroke ○ Cortical function ○ Embolic stroke ○ Cerebral vasculature ○ Haemorrhagic Stroke ○ Motor and sensory ○ Young stroke pathways ○ Skull compartments ● Neuro-imaging ○ The ventricular system ○ Vision pathways Anatomy 1) The CNS Nervous System - Central Nervous System (CNS): consists of brain and spinal cord - Peripheral Nervous System (PNS): nerves joining CNS to peripheral structures The Brain Grey Matter - dense in nerve cell bodies eg central part of spinal cord and surface of cerebral hemispheres. White Matter - contains nerve processes, often myelinated. Embryonic development Primary Brain Vesicles Secondary Brain Mature Brain vesicles (wk 7) Prosencephalon Telencephalon Cerebral hemisphere (forebrain) Diencephalon Thalamus Mesencephalon Mesencephalon Midbrain Rhombencephalon Metencephalon Pons, cerebellum (hindbrain) Myelencephalon Medulla oblongata Anatomy 2) Cortical Function Frontal Lobe - Lies anterior to central sulcus - Precentral gyrus: primary motor cortex - Inferior frontal gyrus of the dominant hemisphere is the motor speech area - Broca’s area. - Middle frontal gyrus: Frontal eye field, controls voluntary conjugate deviation of the eyes when scanning, causes eye deviation towards the side of lesion. - Higher functions: intellectual, judgemental, predictive faculties, planning of behaviour The clinical picture Parietal Lobe - Postcentral gyrus: primary somatosensory cortex - Inferior visual field loss - Parietal association cortex - Sensory information and conscious awareness - Interfaces between visual and auditory association cortices. - Dominant lobe: inability to name objects (anomia), loss of literacy, inability to read (alexia), to write (agraphia) and to calculate (acalculia) - right parietal lobe: constructional apraxia The clinical picture Mrs Z, inability finding her jeans pocket, bumping into doors around corners and some right arm weakness. -

Altered Diffusion in the Frontal Lobe in Parkinson Disease
Altered Diffusion in the Frontal Lobe in Parkinson ORIGINAL RESEARCH Disease A.T. Karagulle Kendi BACKGROUND AND PURPOSE: Parkinson disease (PD) is characterized by basal ganglia abnormalities. S. Lehericy However, there are neurodegenerative changes in PD that extend beyond the basal ganglia and that are not sufficiently evaluated with standard MR imaging. The aim of this study was to characterize M. Luciana whole-brain gray matter (GM) and white matter (WM) changes in PD by using diffusion tensor imaging K. Ugurbil (DTI). P. Tuite MATERIALS AND METHODS: Thirteen control and 12 subjects with nondemented PD were examined by using DTI and 3D anatomic T1-weighted images. Statistical parametric mapping analyses of DTI and anatomic images were performed. Patients were evaluated with a variety of neurocognitive measures and the Unified Parkinson’s Disease Rating Scale (UPDRS) OFF (cessation of medication) and ON (taking medications as normal) their antiparkinsonian medications. RESULTS: The PD participants had dopa-responsive features as ascertained by the UPDRS OFF versus ON medications and had no cognitive impairment. Decreased fractional anisotropy (FA) was observed in subjects with PD bilaterally in the frontal lobes, including the supplementary motor area, the presupplementary motor area, and the cingulum. There were no significant differences in mean diffusivity or GM/WM attenuation between PD subjects and controls. CONCLUSION: Statistical parametric mapping analysis of DTI showed changes in FA in frontal areas without volume loss. These results confirm that the neurodegenerative process extends beyond the basal ganglia in PD. arkinson disease (PD) is a neurodegenerative disorder voxel-based DTI provides information on the orientation of Pcharacterized by bradykinesia, rigidity, resting tremor, diffusion in WM fiber bundles. -

Regional Increases of Cortical Thickness in Untreated, First-Episode
OPEN Citation: Transl Psychiatry (2014) 4, e378; doi:10.1038/tp.2014.18 © 2014 Macmillan Publishers Limited All rights reserved 2158-3188/14 www.nature.com/tp ORIGINAL ARTICLE Regional increases of cortical thickness in untreated, first- episode major depressive disorder L Qiu1,2,7,SLui1,7, W Kuang3, X Huang1,JLi3,JLi4, J Zhang1, H Chen4, JA Sweeney5,6 and Q Gong1 The large majority of structural MRI studies of major depressive disorder (MDD) investigated volumetric changes in chronic medicated patients in whom course of illness and treatment effects may impact anatomic measurements. Further, in few studies, separate measurements of cortical thickness and surface area have been performed that reflect different neurobiological processes regulated by different genetic mechanisms. In the present study, we investigated both cortical thickness and surface area in first- episode, treatment-naïve, mid-life MDD to elucidate the core pathophysiology of this disease and its early impact on the brain. We observed increased cortical thickness in the right hemisphere, including medial orbitofrontal gyrus, pars opercularis, rostral middle frontal gyrus and supramarginal gyrus. Increased thickness of rostral middle frontal gyrus was negatively related with depression severity on the Hamilton Depression Rating Scale. Furthermore, MDD patients showed significantly increased associations in cortical thickness measurements among areas where increased cortical thickness was observed. Analysis of pial area revealed a trend toward increased surface area in the left parahippocampal gyrus in MDD. To permit comparison of our data with those of previous gray matter volume studies, voxel-based morphometry was performed. That analysis revealed significantly increased gray matter volume in left paracentral lobule, left superior frontal gyrus, bilateral cuneus and thalamus which form limbic- cortico–striato–pallido–thalamic loops. -

Subregions of the Human Superior Frontal Gyrus and Their Connections
NeuroImage 78 (2013) 46–58 Contents lists available at SciVerse ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Subregions of the human superior frontal gyrus and their connections Wei Li a,1, Wen Qin a,1, Huaigui Liu a, Lingzhong Fan b, Jiaojian Wang b, Tianzi Jiang b,⁎, Chunshui Yu a,⁎⁎ a Department of Radiology, Tianjin Medical University General Hospital, Tianjin 300052, PR China b LIAMA Center for Computational Medicine, National Laboratory of Pattern Recognition, Institute of Automation, Chinese Academy of Sciences, Beijing 100190, PR China article info abstract Article history: The superior frontal gyrus (SFG) is located at the superior part of the prefrontal cortex and is involved in a variety Accepted 5 April 2013 of functions, suggesting the existence of functional subregions. However, parcellation schemes of the human SFG Available online 13 April 2013 and the connection patterns of each subregion remain unclear. We firstly parcellated the human SFG into the anteromedial (SFGam), dorsolateral (SFGdl), and posterior (SFGp) subregions based on diffusion tensor Keywords: tractography. The SFGam was anatomically connected with the anterior and mid-cingulate cortices, which are Diffusion tensor imaging critical nodes of the cognitive control network and the default mode network (DMN). The SFGdl was connected Superior frontal gyrus Tractography with the middle and inferior frontal gyri, which are involved in the cognitive execution network. The SFGp was Parcellation connected with the precentral gyrus, caudate, thalamus, and frontal operculum, which are nodes of the motor Resting-state control network. Resting-state functional connectivity analysis further revealed that the SFGam was mainly Fingerprint correlated with the cognitive control network and the DMN; the SFGdl was correlated with the cognitive execution network and the DMN; and the SFGp was correlated with the sensorimotor-related brain regions.