Coal Power Station
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GRMB Annual Report 2017-18
Government of India Ministry of Water Resources, RD & GR Godavari River Management Board ANNUAL REPORT 2017-18 GODAVARI BASIN – Dakshina Ganga Origin Brahmagiri near Trimbakeshwar, Nasik Dist., Maharashtra Geographical Area 9.50 % of Total GA of India Area & Location Latitude - 16°19’ to 22°34’ North Longitude – 73°24’ to 83° 4’ East Boundaries West: Western Ghats North: Satmala hills, the Ajanta range and the Mahadeo hills East: Eastern Ghats & the Bay of Bengal South: Balaghat & Mahadeo ranges stretching forth from eastern flank of the Western Ghats & the Anantgiri and other ranges of the hills and ridges separate the Gadavari basin from the Krishna basin. Catchment Area 3,12,812 Sq.km Length of the River 1465 km States Maharashtra (48.6%), Telangana (18.8%), Andhra Pradesh (4.5%), Chhattisgarh (10.9%), Madhya Pradesh (10.0%), Odisha (5.7%), Karnataka (1.4%) and Puducherry (Yanam) and emptying into Bay of Bengal Length in AP & TS 772 km Major Tributaries Pravara, Manjira, Manair – Right side of River Purna, Pranhita, Indravati, Sabari – Left side of River Sub- basins Twelve (G1- G12) Dams Gangapur Dam, Jayakwadi dam, Vishnupuri barrage, Ghatghar Dam, Upper Vaitarna reservoir, Sriram Sagar Dam, Dowleswaram Barrage. Hydro power stations Upper Indravati 600 MW Machkund 120 MW Balimela 510 MW Upper Sileru 240 MW Lower Sileru 460 MW Upper Kolab 320 MW Pench 160 MW Ghatghar pumped storage 250 MW Polavaram (under 960 MW construction) ANNUAL REPORT 2017-18 GODAVARI RIVER MANAGEMENT BOARD 5th Floor, Jalasoudha, Errum Manzil, Hyderabad- 500082 FROM CHAIRMAN’S DESK It gives me immense pleasure to present the Annual Report of Godavari River Management Board (GRMB) for the year 2017-18. -

Indian Power Sector and Contribution of Maharashtra
Vol-3 Issue-1 2017 IJARIIE-ISSN(O)-2395-4396 INDIAN POWER SECTOR AND CONTRIBUTION OF MAHARASHTRA Kale M. L1., Mate A. K., Narwade V. B., Vharkate C. B., Rathod N.R. Kale M.L., Lecturer, Mechanical Engg. Department, M.S.Poly, Beed, MH, India Mate A. K., Lecturer, Mechanical Engg. Department, M.S.Poly, Beed, MH, India Narwade V. B., Lecturer, Mechanical Engg. Department, M.S.Poly, Beed, MH, India Vharkate C. B., Lecturer, Mechanical Engg. Department, M.S.Poly, Beed, MH, India Rathod N. R., Lecturer, Mechanical Engg. Department, M.S.Poly, Beed, MH, India ABSTRACT Power Sector in India has grown significantly from independence both in the installed electricity generating capacity and transmission & distribution (T&D) system. The total power generating capacity of (utilities & non utilities) has increased from meager 1362 MW in 1947 to 267 GW at the end of March, 2015. The per capita electricity consumption which was mere 16.3 kWh in1947 has increased to 1010 KW h in 2014-15. With a production of 1,031 TWh. India is the third largest producer and fourth largest consumer of electricity in the world. It has fifth largest installed capacity in the world. Maharashtra plays very vital role in the evolution of the Indian power sector. As of 2012, Maharashtra was the largest power generating state in India, with installed electricity generation capacity with 26,838 MW. The state forms a major constituent of the western grid of India, which now comes under the North, East, West and North Eastern (NEWNE) grids of India. Maharashtra Power Generation Company controls and runs thermal power plants. -

Annual Report 2 0 1 2 - 1 3
Annual Report 2 0 1 2 - 1 3 Ministry of Power Government of India Shram Shakti Bhawan, Rafi Marg, New Delhi-110 001 Website : www.powermin.nic.in Shri Pranab Mukherjee, Hon’ble President of India with Shri Jyotiraditya M. Scindia, Hon’ble Union Minister of State for Power (Independent Charge) at the National Energy Conservation Day function CONTENTS Sl. No. Chapter Page No. (s) 1. Performance Highlights 5 2. Organisational Set Up and Functions of the Ministry of Power 9 3. Capacity Addition Programme in the XIIth Plan 11 4. Generation & Power Supply Position 23 5. Status of Ultra Mega Power Projects 35 6. Transmission 37 7. Status of Power Sector Reforms 41 8. Rural Electrification Programme 43 9. Re-Structured Accelerated Power Development and Reforms Programme (R-APDRP) 45 10. Energy Conservation 49 11. Renovation and Modernisation of Thermal Power Stations 53 12. Private Sector Participation in Power Sector 57 13. International Cooperation 59 14. Power Development Activities in North-Eastern Region 67 15. Central Electricity Authority 75 16. Central Electricity Regulatory Commission (CERC) 79 17. Appellate Tribunal for Electricity (APTEL) 83 Public Sector Undertakings: 18 NTPC Limited 85 19. NHPC Limited 105 20. Power Grid Corporation of India Ltd. (PGCIL) 111 21. Power Finance Corporation Ltd. (PFC) 115 22. Rural Electrification Corporation Ltd. (REC) 125 23. North Eastern Electric Power Corporation Limited (NEEPCO) 133 Joint Venture Corporations : 24. SJVN Limited (SJVNL) 135 25. THDC India Limited (THDCIL) 139 Statutory Bodies : 26. Damodar Valley Corporation (DVC) 143 27. Bhakra Beas Management Board (BBMB) 149 28. Bureau of Energy Efficiency (BEE) 155 Autonomous Bodies : 29. -

2 Designation 3 Conservation Area Boundary 4 Brief
1.9 In line with the guidance given by both the 2 DESIGNATION Government and English Heritage, therefore, this conservation area profile will aim to define the 2.1 The Sands End Conservation Area was character of the conservation area on the basis of designated in 1991. The conservation area was an analysis of all or some of the following designated because of the importance of criteria:- protecting the riverside from unsympathetic development and to encourage the preservation the origins and development of the street and enhancement of the riverside itself, ensuring patterns, the lie of the land; that new development is of a good and archaeological significance and potential of appropriate design. the area, including any scheduled monuments; the architectural and historic quality, 3 CONSERVATION AREA character and coherence of the buildings, both BOUNDARY listed and unlisted, and the contribution which they make to the special interest of the area; 3.1 The area is in the southernmost part of the the character and hierarchy of spaces, and borough, fronting and incorporating the River townscape quality; Thames between the Chelsea Railway Bridge and the Hurlingham Conservation Area. prevalent and traditional building materials for buildings, walls and surfaces; 3.2 To the north and west, the conservation area the contribution made to the character of boundary extends from the railway bridge at the area by greens or green spaces, trees, hedges Battersea Reach along Townmead Road and and other natural or cultivated elements; Carnwath Road to Broomhouse Lane. This includes the slipway to the river at Broomhouse the prevailing (or former) uses within the Dock. -

Bankside Power Station: Planning, Politics and Pollution
BANKSIDE POWER STATION: PLANNING, POLITICS AND POLLUTION Thesis submitted for the degree of Doctor of Philosophy at the University of Leicester by Stephen Andrew Murray Centre for Urban History University of Leicester 2014 Bankside Power Station ii Bankside Power Station: Planning, Politics and Pollution Stephen Andrew Murray Abstract Electricity has been a feature of the British urban landscape since the 1890s. Yet there are few accounts of urban electricity undertakings or their generating stations. This history of Bankside power station uses government and company records to analyse the supply, development and use of electricity in the City of London, and the political, economic and social contexts in which the power station was planned, designed and operated. The close-focus adopted reveals issues that are not identified in, or are qualifying or counter-examples to, the existing macro-scale accounts of the wider electricity industry. Contrary to the perceived backwardness of the industry in the inter-war period this study demonstrates that Bankside was part of an efficient and profitable private company which was increasingly subject to bureaucratic centralised control. Significant decision-making processes are examined including post-war urban planning by local and central government and technological decision-making in the electricity industry. The study contributes to the history of technology and the environment through an analysis of the technologies that were proposed or deployed at the post-war power station, including those intended to mitigate its impact, together with an examination of their long-term effectiveness. Bankside made a valuable contribution to electricity supplies in London until the 1973 Middle East oil crisis compromised its economic viability. -

Company Profile R O B E R T S
RobeR ts & s chaefeR c ompany Company p rofile H o W THe WorlD proC e SSe S i TS reS o U r C e S ROBERTS & SCHAEFER COMPANY PRESENTATION OF GENERAL QUALIFICATIONS TO PERFORM PROFESSIONAL ENGINEERING AND www.r-s.com CONSTRUCTION SERVICES Chicago Pittsburgh Indonesia Poland 222 South Riverside Plaza 4412 Route 66 Sequis Center; 7th Floor ul. Bojkowska 37 Chicago, IL 60606-3986 Apollo, Pennsylvania 15613 JL Jenderal Duirman KAV 71 Gliwice, Poland 44-100 TEL: 312-236-7292 TEL: 801-984-0900 Jakarta, Indonesia 12190 TEL: +48-32-461-2722 FAX: 312-726-2872 FAX: 801-984-0909 TEL: +62 (0) 21-252-4177 FAX: +48-32-461-2720 Email: [email protected] Email: [email protected] FAX: +62 (0) 21-252-4138 Email: [email protected] Email: [email protected] Salt Lake City Australia 10150 South Centennial Parkway Level 11, 199 Grey Street, India Sandy, Utah 84070 South Bank 20, White House, C.G.Road TEL: 801-984-0900 Qld 4101 Australia Ahmedabad, India 380006 FAX: 801-984-0909 TEL: +617 3234 9555 TEL: 079-40328000 Email: [email protected] FAX: +617 3234 9595 FAX: 079-40328001 Email: [email protected] Email: [email protected] HOW THE WORLD PROCESSES ITS RESOURCES ROBERTS & SCHAEFER COMPANY www.r-s.com CONTENTS Who We Are A Team of Expertise Coal Fuel Handling Pet Coke and Alternative Fuel Handling Limestone and Gypsum (FGD) Handling Coal Preparation and Handling Metals and Minerals Processing and Handling Aggregate, Cement and Sand Handling Port and Marine Projects Specialty Projects Another Look Project Management & Execution Summary HOW THE WORLD PROCESSES ITS RESOURCES PREFACE Roberts & Schaefer Company (R&S) provides a wide range of services from complete turnkey design/build responsibility on domestic and international multi-million dollar facilities to engineering-only and feasibility studies. -
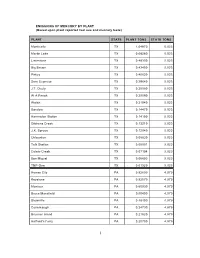
EMISSIONS of MERCURY by PLANT (Based Upon Plant Reported Fuel Use and Mercury Tests)
EMISSIONS OF MERCURY BY PLANT (Based upon plant reported fuel use and mercury tests) PLANT STATE PLANT TONS STATE TONS Monticello TX 1.04870 5.023 Martin Lake TX 0.68280 5.023 Limestone TX 0.48300 5.023 Big Brown TX 0.43450 5.023 Pirkey TX 0.40620 5.023 Sam Seymour TX 0.38640 5.023 J.T. Deely TX 0.25090 5.023 W A Parish TX 0.25080 5.023 Welsh TX 0.21940 5.023 Sandow TX 0.14470 5.023 Harrington Station TX 0.14190 5.023 Gibbons Creek TX 0.13210 5.023 J.K. Spruce TX 0.12040 5.023 Oklaunion TX 0.08839 5.023 Tolk Station TX 0.08001 5.023 Coleto Creek TX 0.07194 5.023 San Miguel TX 0.06693 5.023 TNP-One TX 0.01329 5.023 Hom er City PA 0.92600 4.979 Keystone PA 0.92570 4.979 Montour PA 0.60930 4.979 Bruce Mansfield PA 0.50400 4.979 Shawville PA 0.46400 4.979 Conemaugh PA 0.24730 4.979 Brunner Island PA 0.21820 4.979 Hatfield's Ferry PA 0.20700 4.979 1 EMISSIONS OF MERCURY BY PLANT (Based upon plant reported fuel use and mercury tests) PLANT STATE PLANT TONS STATE TONS Armstrong PA 0.15340 4.979 Cheswick PA 0.11860 4.979 Sunbury PA 0.11810 4.979 New Castle PA 0.10430 4.979 Portland PA 0.06577 4.979 Johnsonburg Mill PA 0.04678 4.979 Titus PA 0.03822 4.979 Cambria CoGen PA 0.03499 4.979 Colver Power Project PA 0.03459 4.979 Elrama PA 0.02900 4.979 Seward PA 0.02633 4.979 Martins Creek PA 0.02603 4.979 Hunlock Power Station PA 0.02580 4.979 Eddystone PA 0.02231 4.979 Mitchell (PA) PA 0.01515 4.979 AES BV Partners Beaver Valley PA 0.01497 4.979 Cromby Generating Station PA 0.00086 4.979 Northampton Generating Company L.P. -

Towards Sustainable Power for Maharashtra Electricity Blueprint
Towards Sustainable Power for Maharashtra Electricity Blueprint Rupali Ghate – GreenEarth Social Development Consulting Pvt. Ltd. March 2013 l Version 2 - Draft for Approval © Maharashtra NavNirman Sena Table of Contents List of Tables ............................................................................................................................... 3 List of Figures .............................................................................................................................. 6 Abbreviations and Acronyms ...................................................................................................... 8 Units .......................................................................................................................................... 11 Overview ....................................................................................................................................... 12 India Power Scenario ................................................................................................................ 13 Maharashtra Power Scenario ................................................................................................... 30 Problems ....................................................................................................................................... 58 Load shedding ........................................................................................................................... 59 Electricity Tariff and Subsidies ................................................................................................. -

Sands End Revisited a Local History Guide
SANDS END REvisitED A Local HISToRy GuIDE We found useful information at the Archive and Local History centre in Hammersmith. There were people that came into school and helped us with our research because they had been residents in Sands End or pupils at Langford many years ago. We also went to Elizabeth Barnes Court Old People’s home to hear more stories and memories of people who knew Sands End in the past. Some of the places in Sands End are named after people that lived here, for example Bagley’s Lane, is named after the Bagley family, locally well known for market gardening and were called the ‘Kings of Fulham’. Elaysha This local history guide about the Sands End area, has been compiled by Y6 from Langford Primary school. Working in partnership with Hammersmith and Fulham Urban Studies Centre, LBHF’s Archives and Local History Centre, and local interest group Sands End Revisited, Oak class became ‘History Detectives’ and were taken back in time to discover the stories of Sands End and the changes that have taken place over the years. By visiting local landmarks, such as the Community Centre on Broughton Road - which was once the old Sunlight laundry - examining old photos at the Archives, and sharing the memories of former local residents and pupils of Langford School, the children discovered a wealth of information, learnt about the changes that have taken place over the years and tried to uncover any secrets about the local area. The stories presented here are a mixture of fact, personal experience, and maybe a little bit of urban myth thrown in to really get you thinking! Thanks to Heritage Lottery Fund for the grant to produce the project. -

Patel Engineering Limited
PROSPECTUS Please read Section 60B of the Companies Act, 1956. Dated May 15, 2006 100% Book Built Issue PATEL ENGINEERING LIMITED (Our Company was incorporated as “Patel Engineering Company Limited” on April 2, 1949 at Mumbai under the Companies Act, 1913 with the Registration No. 7039 of 1949- 50. The name of our Company was changed to “Patel Engineering Limited” with effect from December 9, 1999. For details of changes to our Registered Office, please refer to the chapter titled “History and Other Corporate Matters” on page 78 of this Prospectus. Registered Office: Patel Estate Road, Jogeshwari (West), Mumbai – 400 102. Tel: +91 22 2678 2916 • Fax: +91 22 2678 2455. Contact Person: Ms. Shobha Shetty • E-mail: [email protected] • Website: www.pateleng.com PUBLIC ISSUE OF 9,659,090 EQUITY SHARES OF RE. 1 EACH AT A PRICE OF RS. 440 PER EQUITY SHARE (INCLUDING SHARE PREMIUM OF RS. 439 PER EQUITY SHARE) FOR CASH AGGREGATING RS. 4,250 MILLION (HEREINAFTER REFERRED TO AS THE “ISSUE”). THE ISSUE COMPRISES A NET ISSUE TO THE PUBLIC OF UP TO 9,176,135 EQUITY SHARES OF RE. 1 EACH AGGREGATING TO RS. 4,037.50 MILLION (“NET ISSUE”) AND A RESERVATION FOR EXISTING RETAIL SHAREHOLDERS OF THE COMPANY OF UP TO 482,955 EQUITY SHARES OF RE. 1 EACH (“EXISTING RETAIL SHAREHOLDERS’ RESERVATION PORTION”) AGGREGATING TO RS. 212.50 MILLION. THE ISSUE WILL CONSTITUTE 16.2% OF THE FULLY DILUTED POST ISSUE PAID-UP CAPITAL OF THE COMPANY. THE ISSUE PRICE IS RS. 440 PER EQUITY SHARE THE FACE VALUE OF THE EQUITY SHARES IS Re. -

Fly ASH–A Boon for Concrete Raj Premani B.Tech Department of Civil Engineering Career Point University, Kota, Rajasthan, India
ISSN XXXX XXXX © 2017 IJESC Research Article Volume 7 Issue No.3 Fly ASH–A Boon for Concrete Raj Premani B.Tech Department of Civil Engineering Career Point University, Kota, Rajasthan, India Abstract: Electricity is a critical input needed for the development of any country. In India, coal is the main source of generating power at present and likely to remain in the future too. India is the world’s second largest producer after china and fourth largest consumer of electricity. In India, 60% of the electricity is generated through coal fired thermal power plant and in this production of electricity 70% of the coal produced in India is used. India need more energy to meet the rapidly growing demand, hence the use of coal will continue to increase. Electricity produced through coal is cheap, reliable and most widely used all over the world but it has much bad impact on the environment and human health, the main reason of concern is dispos al of fly ash. In the past fly ash was generally released into the atmosphere, but this leads to air pollution but later on it was collected by pollution control equipment like electrostatic precipitator prior to its release to control the air pollution. So this leads to the problem of fly ash disposal. Fly ash are generally deposited in ash ponds which require large area but this leads to bad impact on environment and human health so rec ycling of fly ash has become an increasing concern. Keywords: fly ash, fly ash disposal, electricity, cement, concrete, bricks, PPC. -

Return Receipt Requested the Hon. Regina Mccarthy, Administrator US
January 14, 2016 Via Certified Mail - Return Receipt Requested The Hon. Regina McCarthy, Administrator U.S. Environmental Protection Agency Ariel Rios Building 1200 Pennsylvania Avenue, N.W. Mail Code: 1101A Washington, DC 20460 Via Certified Mail - Return Receipt Requested Heather McTeer Toney, Regional Administrator U.S. Environmental Protection Agency, Region 4 61 Forsyth Street, SW Atlanta, GA 30303-3104 Via Certified Mail - Return Receipt Requested Mr. Robert J. Martineau, Jr., Commissioner Tennessee Department of Environment and Conservation William R. Snodgrass Tennessee Tower 312 Rosa L. Parks Avenue, 2nd Floor Nashville, TN 37243 Via Certified Mail - Return Receipt Requested Mr. Bill Johnson, President and Chief Executive Officer Tennessee Valley Authority 400 West Summit Hill Drive Knoxville, TN 37902-1499 RE: 60-Day Notice of Intent to Sue, 33 U.S.C. § 1365, for Violations of the Clean Water Act by Tennessee Valley Authority–TVA Cumberland Fossil Plant (CUF), NPDES No. TN0005789 To Whom It May Concern: This letter is to notify the United States Environmental Protection Agency (“EPA”), the Tennessee Department of Environment and Conservation (“TDEC”), and the Tennessee Valley Authority (“TVA”) of ongoing violations of the Clean Water Act (“CWA”) at the Cumberland Fossil Plant (“the Cumberland Plant”) in Cumberland City, Tennessee, owned and operated by 1371158_2 TVA-Cumberland Fossil Plant January 14, 2016 Page 2 of 35 TVA. The Sierra Club (“the Conservation Group”) and its members have identified serious and ongoing unpermitted violations of the CWA at the Cumberland Plant. TVA has caused and continues to cause unauthorized point source discharges to Tennessee waters and navigable waters of the U.S., and to cause unpermitted pollutant discharges to flow from the coal ash disposal areas at the Cumberland Plant directly into the Cumberland River, as well as into groundwater that is hydrologically connected to the Cumberland River.