DISSERTATION O Attribution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coal Power Station
Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org Coal Power Station ( Fly Ash , Bottom Ash & Flue Gas Desulfurization ) BY Tarek Ismail Kakhia 1 Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org Contents No Item Page 1 Fossil - fuel power station 3 2 Chimney 11 3 Fly Ash -1 21 4 Fly Ash -2 44 5 Electrostatic precipitator 44 4 Bottom Ash 52 7 Flue - Gas Desulfurization ( FGD ) 53 8 Flue-gas emissions from fossil-fuel combustion 44 1 Flue - gas stack 47 10 Calcium Sulfite 72 11 Calcium bi sulfite 73 12 Calcium sulfate 74 2 Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org Fossil - fuel power station Contents 1 Basic concepts o 1.1 Heat into mechanical energy 2 Fuel transport and delivery 3 Fuel processing 4 Steam - electric 5 Gas turbine plants 6 Reciprocating engines 7 Environmental impacts o 7.1 Carbon dioxide o 7.2 Particulate matter o 7.3 Radioactive trace elements o 7.4 Water and air contamination by coal ash . 7.4.1 Range of mercury contamination in fish 8 Greening of fossil fuel power plants o 8.1 Low NOx Burners o 8.2 Clean coal 9 Combined heat and power 10 Alternatives to fossil fuel power plants o 10.1 Relative cost by generation source - Introduction : A fossil - fuel power station is a power station that burns fossil fuels such as coal, natural gas or petroleum (oil) to produce electricity. Central station fossil - fuel power plants are designed on a large scale for continuous operation. In many countries, such plants provide most of the electrical energy used. -

Company Profile R O B E R T S
RobeR ts & s chaefeR c ompany Company p rofile H o W THe WorlD proC e SSe S i TS reS o U r C e S ROBERTS & SCHAEFER COMPANY PRESENTATION OF GENERAL QUALIFICATIONS TO PERFORM PROFESSIONAL ENGINEERING AND www.r-s.com CONSTRUCTION SERVICES Chicago Pittsburgh Indonesia Poland 222 South Riverside Plaza 4412 Route 66 Sequis Center; 7th Floor ul. Bojkowska 37 Chicago, IL 60606-3986 Apollo, Pennsylvania 15613 JL Jenderal Duirman KAV 71 Gliwice, Poland 44-100 TEL: 312-236-7292 TEL: 801-984-0900 Jakarta, Indonesia 12190 TEL: +48-32-461-2722 FAX: 312-726-2872 FAX: 801-984-0909 TEL: +62 (0) 21-252-4177 FAX: +48-32-461-2720 Email: [email protected] Email: [email protected] FAX: +62 (0) 21-252-4138 Email: [email protected] Email: [email protected] Salt Lake City Australia 10150 South Centennial Parkway Level 11, 199 Grey Street, India Sandy, Utah 84070 South Bank 20, White House, C.G.Road TEL: 801-984-0900 Qld 4101 Australia Ahmedabad, India 380006 FAX: 801-984-0909 TEL: +617 3234 9555 TEL: 079-40328000 Email: [email protected] FAX: +617 3234 9595 FAX: 079-40328001 Email: [email protected] Email: [email protected] HOW THE WORLD PROCESSES ITS RESOURCES ROBERTS & SCHAEFER COMPANY www.r-s.com CONTENTS Who We Are A Team of Expertise Coal Fuel Handling Pet Coke and Alternative Fuel Handling Limestone and Gypsum (FGD) Handling Coal Preparation and Handling Metals and Minerals Processing and Handling Aggregate, Cement and Sand Handling Port and Marine Projects Specialty Projects Another Look Project Management & Execution Summary HOW THE WORLD PROCESSES ITS RESOURCES PREFACE Roberts & Schaefer Company (R&S) provides a wide range of services from complete turnkey design/build responsibility on domestic and international multi-million dollar facilities to engineering-only and feasibility studies. -
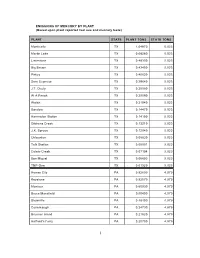
EMISSIONS of MERCURY by PLANT (Based Upon Plant Reported Fuel Use and Mercury Tests)
EMISSIONS OF MERCURY BY PLANT (Based upon plant reported fuel use and mercury tests) PLANT STATE PLANT TONS STATE TONS Monticello TX 1.04870 5.023 Martin Lake TX 0.68280 5.023 Limestone TX 0.48300 5.023 Big Brown TX 0.43450 5.023 Pirkey TX 0.40620 5.023 Sam Seymour TX 0.38640 5.023 J.T. Deely TX 0.25090 5.023 W A Parish TX 0.25080 5.023 Welsh TX 0.21940 5.023 Sandow TX 0.14470 5.023 Harrington Station TX 0.14190 5.023 Gibbons Creek TX 0.13210 5.023 J.K. Spruce TX 0.12040 5.023 Oklaunion TX 0.08839 5.023 Tolk Station TX 0.08001 5.023 Coleto Creek TX 0.07194 5.023 San Miguel TX 0.06693 5.023 TNP-One TX 0.01329 5.023 Hom er City PA 0.92600 4.979 Keystone PA 0.92570 4.979 Montour PA 0.60930 4.979 Bruce Mansfield PA 0.50400 4.979 Shawville PA 0.46400 4.979 Conemaugh PA 0.24730 4.979 Brunner Island PA 0.21820 4.979 Hatfield's Ferry PA 0.20700 4.979 1 EMISSIONS OF MERCURY BY PLANT (Based upon plant reported fuel use and mercury tests) PLANT STATE PLANT TONS STATE TONS Armstrong PA 0.15340 4.979 Cheswick PA 0.11860 4.979 Sunbury PA 0.11810 4.979 New Castle PA 0.10430 4.979 Portland PA 0.06577 4.979 Johnsonburg Mill PA 0.04678 4.979 Titus PA 0.03822 4.979 Cambria CoGen PA 0.03499 4.979 Colver Power Project PA 0.03459 4.979 Elrama PA 0.02900 4.979 Seward PA 0.02633 4.979 Martins Creek PA 0.02603 4.979 Hunlock Power Station PA 0.02580 4.979 Eddystone PA 0.02231 4.979 Mitchell (PA) PA 0.01515 4.979 AES BV Partners Beaver Valley PA 0.01497 4.979 Cromby Generating Station PA 0.00086 4.979 Northampton Generating Company L.P. -

Return Receipt Requested the Hon. Regina Mccarthy, Administrator US
January 14, 2016 Via Certified Mail - Return Receipt Requested The Hon. Regina McCarthy, Administrator U.S. Environmental Protection Agency Ariel Rios Building 1200 Pennsylvania Avenue, N.W. Mail Code: 1101A Washington, DC 20460 Via Certified Mail - Return Receipt Requested Heather McTeer Toney, Regional Administrator U.S. Environmental Protection Agency, Region 4 61 Forsyth Street, SW Atlanta, GA 30303-3104 Via Certified Mail - Return Receipt Requested Mr. Robert J. Martineau, Jr., Commissioner Tennessee Department of Environment and Conservation William R. Snodgrass Tennessee Tower 312 Rosa L. Parks Avenue, 2nd Floor Nashville, TN 37243 Via Certified Mail - Return Receipt Requested Mr. Bill Johnson, President and Chief Executive Officer Tennessee Valley Authority 400 West Summit Hill Drive Knoxville, TN 37902-1499 RE: 60-Day Notice of Intent to Sue, 33 U.S.C. § 1365, for Violations of the Clean Water Act by Tennessee Valley Authority–TVA Cumberland Fossil Plant (CUF), NPDES No. TN0005789 To Whom It May Concern: This letter is to notify the United States Environmental Protection Agency (“EPA”), the Tennessee Department of Environment and Conservation (“TDEC”), and the Tennessee Valley Authority (“TVA”) of ongoing violations of the Clean Water Act (“CWA”) at the Cumberland Fossil Plant (“the Cumberland Plant”) in Cumberland City, Tennessee, owned and operated by 1371158_2 TVA-Cumberland Fossil Plant January 14, 2016 Page 2 of 35 TVA. The Sierra Club (“the Conservation Group”) and its members have identified serious and ongoing unpermitted violations of the CWA at the Cumberland Plant. TVA has caused and continues to cause unauthorized point source discharges to Tennessee waters and navigable waters of the U.S., and to cause unpermitted pollutant discharges to flow from the coal ash disposal areas at the Cumberland Plant directly into the Cumberland River, as well as into groundwater that is hydrologically connected to the Cumberland River. -

Power Plants and Mercury Pollution Across the Country
September 2005 Power Plants and Mercury Pollution Across the Country NCPIRG Education Fund Made in the U.S.A. Power Plants and Mercury Pollution Across the Country September 2005 NCPIRG Education Fund Acknowledgements Written by Supryia Ray, Clean Air Advocate with NCPIRG Education Fund. © 2005, NCPIRG Education Fund The author would like to thank Alison Cassady, Research Director at NCPIRG Education Fund, and Emily Figdor, Clean Air Advocate at NCPIRG Education Fund, for their assistance with this report. To obtain a copy of this report, visit our website or contact us at: NCPIRG Education Fund 112 S. Blount St, Ste 102 Raleigh, NC 27601 (919) 833-2070 www.ncpirg.org Made in the U.S.A. 2 Table of Contents Executive Summary...............................................................................................................4 Background: Toxic Mercury Emissions from Power Plants ..................................................... 6 The Bush Administration’s Mercury Regulations ................................................................... 8 Findings: Power Plant Mercury Emissions ........................................................................... 12 Power Plant Mercury Emissions by State........................................................................ 12 Power Plant Mercury Emissions by County and Zip Code ............................................... 12 Power Plant Mercury Emissions by Facility.................................................................... 15 Power Plant Mercury Emissions by Company -

Georgia Power Company's Applications for Solid Waste
August 5, 2019 Via U.S. Mail and email Hon. Richard E. Dunn Mr. Chuck Mueller Director Branch Chief Environmental Protection Division Land Protection Branch Georgia Dept. of Natural Resources Environmental Protection Division 2 Martin Luther King Jr. Drive, SE Georgia Dept. of Natural Resources Floyd Towers EastSuite 1456 2 Martin Luther King Jr. Drive, SE Atlanta, GA 30334-9000 Suite 1054 East Floyd Tower c/o Atlanta, GA 30334-9000 Dir. of Legal Services, Laura Williams, Esq., [email protected] [email protected] RE: Georgia Power Company’s applications for solid waste handling permits for closure of coal ash ponds at Plant Scherer (AP-1), Plant Wansley (AP-1), Plant Yates (AP-B’ and AP-3 “Ash Management Area” and R6 landfill), Plant McDonough (AP-1, AP-3, AP-4), and Plant Hammond (AP-3) Dear Director Dunn and Branch Chief Mueller: On our own behalf and on behalf of the Altamaha Riverkeeper, Chattahoochee Riverkeeper, Inc., and Coosa River Basin Initiative, the Southern Environmental Law Center writes to request that EPD deny Georgia Power Company’s solid waste permit applications to dispose of coal combustion residuals (CCR or coal ash) in place at the above five plants. Denial of these permits is required for numerous reasons, the most glaring of which is that Georgia Power’s closure plans are illegal under both Georgia and federal solid waste laws. In exercising permitting authority over coal ash disposal for the first time in Georgia, EPD has a historic opportunity, and a duty, to assure that coal ash handling and disposal in Georgia “does not adversely affect the health, safety, and well-being of the public” and that such practices “do not degrade the quality of the environment by reason of their location, design, method of operation, or other means ….” O.C.G.A. -

Coal Ash Ponds in Flood Zones
Accidents Waiting to Happen Coal Ash Ponds Put Our Waterways at Risk Accidents Waiting to Happen Coal Ash Ponds Put Our Waterways at Risk Written by: Gideon Weissman Frontier Group John Rumpler Environment America Research & Policy Center April 2018 Acknowledgments Environment America Research & Policy Center and U.S. PIRG Education Fund thank Pat Calvert of the Virginia Conservation Network, Trent Dougherty of the Ohio Environmental Council, Peter Harrison of EarthJustice, and Larissa Liebmann of the Waterkeeper Alliance for their review of drafts of this document, as well as their insights and suggestions. Thanks to Teague Morris, formerly of Frontier Group, for his contributions to earlier drafts of this report, and to Tony Dutzik and Alana Miller of Frontier Group for editorial support. Environment America Research & Policy Center and U.S. PIRG Education Fund thank the Park Foundation for making this report possible. The authors bear responsibility for any factual errors. The recommendations are those of Environment America Research & Policy Center and U.S. PIRG Education Fund. The views expressed in this report are those of the authors and do not necessarily reflect the views of our funders or those who provided review. 2018 Environment America Research & Policy Center and U.S. PIRG Education Fund Environment America Research & Policy Center is a 501(c)(3) organization. We are dedicated to protecting our air, water and open spaces. We investigate problems, craft solutions, educate the public and decision-makers, and help the public make their voices heard in local, state and national debates over the quality of our environment and our lives. -

Tennessee Valley Authority (TVA) Key Messages, August 2017
Description of document: Tennessee Valley Authority (TVA) Key Messages, August 2017 Requested date: 31-July-2017 Released date: 14-August-2017 Posted date: 21-August-2017 Source of document: FOIA Officer Tennessee Valley Authority 400 W. Summit Hill Drive WT 7D Knoxville, TN 37902-1401 (865) 632-6945 The governmentattic.org web site (“the site”) is noncommercial and free to the public. The site and materials made available on the site, such as this file, are for reference only. The governmentattic.org web site and its principals have made every effort to make this information as complete and as accurate as possible, however, there may be mistakes and omissions, both typographical and in content. The governmentattic.org web site and its principals shall have neither liability nor responsibility to any person or entity with respect to any loss or damage caused, or alleged to have been caused, directly or indirectly, by the information provided on the governmentattic.org web site or in this file. The public records published on the site were obtained from government agencies using proper legal channels. Each document is identified as to the source. Any concerns about the contents of the site should be directed to the agency originating the document in question. GovernmentAttic.org is not responsible for the contents of documents published on the website. Key Messages August 2017 Updated August 10, 2017 Key Messages for August 2017 Contents Allen Combined Cycle Plant Water Energy Efficiency, Renewable and Source ....................................................... 4 Distributed Generation ............................. 19 Allen Combined Cycle Plant Solar Grid Stability ......................................... 20 Generation ................................................. 5 Renewable Energy .............................. -

State of Failure: How States Fail to Protect Our Health and Drinking
ST AT E OF FAILU R E H O W STA TES FA IL TO PRO TECT O UR H EA LTH A ND DRINKING W A TER FRO M TO XIC CO A L A SH 37 CO A L A SH REGULA TO RY PRO GRA M S TH A T PLA CE O UR A IR, W A TER A ND H EA LTH IN DA NGER Principal Authors: Lisa Evans Senior Administrative Counsel Earthjustice Michael Becher Attorney Appalachian Mountain Advocates Bridget Lee Attorney Earthjustice Additional Contributors: Jeff Stant and John Dawes Environmental Integrity Project Appreciation: We thank Sue Sturgis of the Institute for Southern Studies and author of Facing South for the use of her compilation of coal ash damage cases (Appendix 1 of this report). © August 2011 Cover Photo: Coal ash spill in Forward Township, PA, January 2005. STATE OF FAILURE How States Fail to Protect Our Health and Drinking Water from Toxic Coal Ash Introduction: An Unhealthy Union Coal ash is the second largest industrial waste stream in the United States. More than 140 million tons of coal ash, comprised of fly ash, bottom ash, boiler slag and flue gas desulfurization (FGD) sludge, are generated annually by the nation’s coal-fired power plants. Coal ash contains a long list of carcinogenic and neurotoxic chemicals such as arsenic, lead, hexavalent chromium, cadmium and mercury. The toxic brew is stored in more than a thousand unstable ponds and landfills, which are located in nearly every state in the nation. Yet most states don’t have regulations in place to keep these toxic chemicals safely entombed and out of our air and drinking water. -

Outside the Law Restoring Accountability to the Tennessee Valley Authority
Outside the Law RestoRing AccountAbility to the tennessee VAlley AuthoRity Outside the Law RestoRing AccountAbility to the tennessee VAlley AuthoRity enViRonmentAl integRity PRoject December 2009 This report was made possible through a grant from Our Children’s Earth Foundation, www.ocefoundation.org © December 2009, Environmental Integrity Project The environmenTal inTegriTy ProjecT is a nonpartisan, nonprofit organization established in March of 2002 by former EPA enforcement attorneys to advocate for more effective enforcement of environmental laws. The Environmental Integrity Project has three objectives: To analyze how the failure to enforce or imple- ment environmental laws increases pollution and affects the public’s health; to hold federal and state agencies, as well as individual corporations, accountable for failing to enforce or comply with environmental laws; and to help local communities in key states obtain the protection of environmental laws. For more information about Environmental Integrity Project or for additional copies of this report, please visit: www.environmentalintegrity.org. cover PhoTos: Cumberland Fossil Plant, Aerial photo of Kingston Fossil Plant ash spill, and Ash and Water Discharge Pipe, available at TVA.gov and in the public domain under 17 U.S.C. § 105. Photo of home buried in coal ash from the Kingston Fossil Plant ash spill was taken by J. Miles Cary of the Knoxville News Sentinel and used with permission. Layout: www.cuttingedgedc.com contents Executive Summary v Summary v Recommendations vii Introduction -

Kingston Fossil Plant
KINGSTON FOSSIL PLANT Tennessee Valley Authority welcomes you to the Environmental Investigation Plan Kingston Fossil Plant COMMUNITY INFORMATION SESSION KINGSTON FOSSIL PLANT EVENT GUIDE This event is to provide information about the Environmental Investigation Plan (EIP) for the Kingston Fossil Plant. We welcome your feedback and comments on the EIP. The following acronyms appear frequently on the boards: • Environmental Investigation Plan (EIP) • Sampling and Analysis Plan (SAP) • Environmental Assessment Report (EAR) • Environmental Protection Agency (EPA) • Coal Combustion Residuals (CCR) • Long Term Monitoring Sampling and Analysis Plan (LTM SAP) • Tennessee Valley Authority (TVA) • Kingston Recovery Project (KRP) • Tennessee Department of Environment and Conservation (TDEC) The information boards are color-coded according to the technical focus area they cover in the EIP. For instance, if they contain general information General Information regarding the EIP process, the information board will be color-coded in blue. Below are the main areas of technical focus in the EIP, divided by study area. More information on the activities within each area is available at stations around the room. The evaluation of existing site data serves as the foundation to support the additional studies planned for each focus area. Geotechnical Civil/Mapping Hydrogeologic Environmental Activities • Exploratory Drilling • Coal Combustion • Hydrogeologic • Benthic Investigation Residuals Material Investigation Quantity • Slope Stability • Groundwater • -

Final Environmental Assessment Paradise Fossil
FINAL ENVIRONMENTAL ASSESSMENT PARADISE FOSSIL PLANT UNITS 1 AND 2 MERCURY AND AIR TOXICS STANDARDS COMPLIANCE PROJECT Muhlenberg County, Kentucky Prepared by: TENNESSEE VALLEY AUTHORITY Knoxville, Tennessee November 2013 To request further information, contact: Charles P. Nicholson NEPA Interface Tennessee Valley Authority 400 West Summit Hill Drive, WT 11D Knoxville, Tennessee 37902–1499 E–mail: [email protected] Table of Contents Table of Contents 1.0 PURPOSE AND NEED FOR ACTION ..................................................................................... 1 1.1. Introduction ............................................................................................................................... 1 1.2. Purpose and Need .................................................................................................................... 1 1.3. Location and Description .......................................................................................................... 2 1.3.1. Paradise Fossil Plant ......................................................................................................... 2 1.3.2. Existing Coal Generation and Support Facilities ............................................................... 2 1.4. Related Environmental Reviews and Initiatives ....................................................................... 5 1.5. Scope of the Environmental Assessment ................................................................................. 5 1.6. Public and Agency Involvement ..............................................................................................