June 2020 DISCLAIMER
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances" WHO/EMP/RHT/TSN/2018.1
INN Working Document 19.450 04/02/2019 Addendum1 to "The use of stems in the selection of International Nonproprietary names (INN) for pharmaceutical substances" WHO/EMP/RHT/TSN/2018.1 Programme on International Nonproprietary Names (INN) Technologies Standards and Norms (TSN) Regulation of Medicines and other health technologies (RHT) World Health Organization, Geneva © World Health Organization 2019 - All rights reserved. The contents of this document may not be reviewed, abstracted, quoted, referenced, reproduced, transmitted, distributed, translated or adapted, in part or in whole, in any form or by any means, without explicit prior authorization of the WHO INN Programme. This document contains the collective views of the INN Expert Group and does not necessarily represent the decisions or the stated policy of the World Health Organization. Addendum1 to "The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances" - WHO/EMP/RHT/TSN/2018.1 1 This addendum is a cumulative list of all new stems selected by the INN Expert Group since the publication of "The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances" 2018. ------------------------------------------------------------------------------------------------------------ -calcet/-calcet- calcium-sensing receptors (CaSR) agonists cinacalcet (88), etelcalcetide (112), evocalcet (113), tecalcet (87), upacicalcet (118) ------------------------------------------------------------------------------------------------------------ -

Myovant Sciences Ltd. 10K 2021 V1
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended March 31, 2021 or ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from _______ to _______ Commission file number 001-37929 Myovant Sciences Ltd. (Exact name of registrant as specified in its charter) Bermuda 98-1343578 (State or other jurisdiction of incorporation or organization) (I.R.S. Employer Identification No.) Suite 1, 3rd Floor 11-12 St. James’s Square London SW1Y 4LB United Kingdom Not Applicable (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: +44 (207) 400 3351 Securities registered pursuant to Section 12(b) of the Act: Title of each Class Trading Symbol Name of each exchange on which registered Common Shares, $0.000017727 par value per share MYOV New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Obseva SA Reports Consistent Long-Term Findings from Phase 2B EDELWEISS Trial of Linzagolix for Endometriosis-Associated Pain
ObsEva SA Reports Consistent Long-Term Findings from Phase 2b EDELWEISS trial of Linzagolix for Endometriosis-Associated Pain Geneva, Switzerland and Boston, MA – May 3, 2019 – ObsEva SA (NASDAQ: OBSV / SIX: OBSN), a clinical- stage biopharmaceutical company focused on the development and commercialization of novel therapeutics for serious conditions that compromise a woman’s reproductive health and pregnancy, today reported follow-up results from the Phase 2b EDELWEISS clinical trial of its oral gonadotropin releasing hormone (GnRH) receptor antagonist, linzagolix, for the treatment of endometriosis-associated pelvic pain. These new data include 28-week extension study treatment (52 weeks of continuous treatment), as well as the 24-week post treatment follow-up (PTFU) results for patients who did not enter the extension study after completing the initial 24-week treatment period. "We are pleased to report long-term data from the EDELWEISS trial of linzagolix, which show that in patients treated with linzagolix for 52 weeks, pelvic pain response rates are maintained with the 75mg or the 200mg dose. Bone mineral density remains within safe limits. Patients that were followed for 6 months after treatment completion continue to experience pain control, and showed BMD increase,” said Ernest Loumaye, Co-Founder and Chief Executive Officer of ObsEva. “These data further support the long term therapeutic potential of linzagolix and support the currently starting Phase 3 program for the endometriosis indication, as we anticipate initial Phase 3 clinical results later this year from the trial in uterine fibroids.” Overall Pelvic Pain reduction sustained long-term After 52 weeks of treatment, responder rates for Overall Pelvic Pain (OPP)— defined as the proportion of patients experiencing an OPP score reduction >30% from baseline using a verbal rating scale— were 69% for the 75mg once daily dose and 82% for the 200mg/100mg once daily dose. -

EC313-A Tissue Selective SPRM Reduces the Growth and Proliferation of Uterine Fbroids in a Human Uterine Fbroid Tissue Xenograft Model Hareesh B
www.nature.com/scientificreports OPEN EC313-a tissue selective SPRM reduces the growth and proliferation of uterine fbroids in a human uterine fbroid tissue xenograft model Hareesh B. Nair1*, Bindu Santhamma1, Kalarickal V. Dileep2, Peter Binkley3, Kirk Acosta1, Kam Y. J. Zhang 2, Robert Schenken3 & Klaus Nickisch1 Uterine fbroids (UFs) are associated with irregular or excessive uterine bleeding, pelvic pain or pressure, or infertility. Ovarian steroid hormones support the growth and maintenance of UFs. Ulipristal acetate (UPA) a selective progesterone receptor (PR) modulator (SPRM) reduce the size of UFs, inhibit ovulation and lead to amenorrhea. Recent liver toxicity concerns with UPA, diminished enthusiasm for its use and reinstate the critical need for a safe, efcacious SPRM to treat UFs. In the current study, we evaluated the efcacy of new SPRM, EC313, for the treatment for UFs using a NOD-SCID mouse model. EC313 treatment resulted in a dose-dependent reduction in the fbroid xenograft weight (p < 0.01). Estradiol (E2) induced proliferation was blocked signifcantly in EC313-treated xenograft fbroids (p < 0.0001). Uterine weight was reduced by EC313 treatment compared to UPA treatment. ER and PR were reduced in EC313-treated groups compared to controls (p < 0.001) and UPA treatments (p < 0.01). UF specifc desmin and collagen were markedly reduced with EC313 treatment. The partial PR agonism and no signs of unopposed estrogenicity makes EC313 a candidate for the long-term treatment for UFs. Docking studies have provided a structure based explanation for the SPRM activity of EC313. Te unmet need for medical management of uterine fbroids (UFs) has led to the discovery of various novel agents in recent years. -
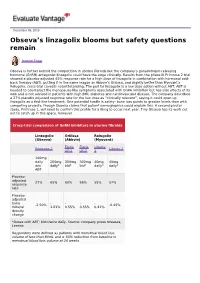
Obseva's Linzagolix Blooms but Safety Questions Remain
December 09, 2019 Obseva’s linzagolix blooms but safety questions remain Joanne Fagg Obseva is further behind the competition in uterine fibroids but the company’s gonadotropin-releasing hormone (GnRH) antagonist linzagolix could have the edge clinically. Results from the phase III Primrose 2 trial showed a placebo-adjusted 65% response rate for a high dose of linzagolix in combination with hormonal add- back therapy (ABT), putting it in the same league as Abbvie’s Orilissa, and slightly better than Myovant’s Relugolix, cross-trial caveats notwithstanding. The pull for linzagolix is a low dose option without ABT; ABT is needed to counteract the menopause-like symptoms associated with GnRH inhibition but has side effects of its own and is not advised in patients with high BMI, diabetes and cardiovascular disease. The company described a 27% placebo adjusted response rate for the low dose as “clinically relevant”, saying it could open up linzagolix as a first-line treatment. One potential hurdle is safety: bone loss points to greater levels than with competing projects, though Obseva claims that patient demographics could explain this. A second pivotal study, Primrose 1, will need to confirm this profile for a filing to occur next year. Tiny Obseva has its work cut out to catch up in this space, however. Cross-trial comparison of GnRH inhibitors in uterine fibroids Linzagolix Orilissa Relugolix (Obseva) (Abbvie) (Myovant) Elaris Elaris Liberty Primrose 2 Liberty 2 UF-1 UF-2 1 100mg daily 200mg 300mg 300mg 40mg 40mg w/o daily* bid* bid* daily* daily* ABT Placebo- adjusted 27% 65% 60% 66% 55% 57% response rate Placebo- adjusted bone - - - - -2.50% -0.45% mineral 1.81% 0.55% 0.55% 0.41% density change *Doses with ABT; bid=twice daily. -

Obseva Needs More to Beat Abbvie in Endometriosis
June 19, 2018 Obseva needs more to beat Abbvie in endometriosis Madeleine Armstrong Obseva, lagging behind its bigger rival Abbvie in the oral gonadotrophin-releasing hormone antagonist space, has a clear mission. The smaller company’s candidate, linzagolix, will need to show best-in-class efficacy if it is to have a chance of competing with Abbvie’s elagolix, which could hit the market this year in its first indication, endometriosis-associated pain. Obseva believes that it has gone some way to proving linzagolix’s superiority with data from the small phase IIb Edelweiss trial in the same disorder, and investors sent the group’s stock up 23% yesterday in response. But a look at how the results stack up against data from elagolix’s pivotal trials suggests some cause for concern (see table below). Of course, cross-trial comparisons should always be treated with caution, and this one is particularly fraught with difficulty, as the studies used different endpoints. But on the most similar measure, response on non- menstrual pain, linzagolix appears to fall short, particularly at the highest dose used, 200mg. Cross-trial comparison of linzagolix and elagolix Placebo 50mg 75mg 100mg 150mg 200mg Linzagolix/OBE2109: Edelweiss (phase IIb, NCT02778399) Response rate (primary endpoint)* 35% 49% 62% 56% - 56% P value - 0.155 0.003 0.039 - 0.034 Response rate, nonmenstrual pain 37% 46% 59% 62% - 48% P value - 0.38 0.017 0.022 - 0.297 Elagolix: Elaris EM-I, Elaris EM-II (phase III, NCT01620528, NCT01931670) Response rate, nonmenstrual pain 37% - - - 50%** 55-58%** *Response defined as reduction of ≥30% in combined menstrual and non-menstrual pelvic pain at week 12; **p<0.003; Source: Company presentations; NEJM paper. -

Manejo De La Paciente Con Miomas Uterinos Y Deseo Reproductivo Guía De Práctica Clínica Basada En La Evidencia Versión Corregida
MANEJO DE LA PACIENTE CON MIOMAS UTERINOS Y DESEO REPRODUCTIVO GUÍA DE PRÁCTICA CLÍNICA BASADA EN LA EVIDENCIA VERSIÓN CORREGIDA Patrocinado por: ÍNDICE Documento de aclaraciones ........................................................................... 5 Introducción ..................................................................................................... 5 Justificación, objetivos y alcance de la guía ................................................ 7 Justificación ................................................................................................. 7 Objetivos ....................................................................................................... 8 Alcance .......................................................................................................... 8 Síntesis de la evidencia e interpretación de las recomendaciones ......... 10 Algoritmos diagnóstico-terapéuticos .......................................................... 25 1. Diagnóstico de los miomas en pacientes con deseo genésico ............ 29 1.1.- Miomas con componente intracavitario.............................................. 30 1.1.1 Rendimiento diagnóstico de la ecografía transvaginal (2D y 3D) .....30 1.1.2 Rendimiento diagnóstico de la histerosonografía ....................... 32 1.1.3 Rendimiento diagnóstico de la histeroscopia frente a la histología ... 34 1.1.4 Rendimiento diagnóstico de la RNM frente a la histología ......... 35 1.2.- Miomas sin componente intracavitario .............................................. -

D Rug U Tilization R Eview B Oard
Wednesday, January 13, 2021 No live meeting scheduled for January. January 2021 will be a packet only meeting. Review Board Review Oklahoma Health Care Authority (OHCA) 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 Drug Utilization The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Michyla Adams, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – January 13, 2021 NOTE: No live January meeting. January 2021 is a packet only meeting. Enclosed are the following items related to the January meeting. Material is arranged in order of the agenda. Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/SoonerCare Opioid Initiative Update – Appendix B Annual Review of Gonadotropin Releasing Hormone (GnRH) Medications and 30-Day Notice to Prior Authorize Fensolvi® (Leuprolide Acetate) and Oriahnn™ (Elagolix/Estradiol/Norethindrone and Elagolix) – Appendix C Annual Review of Antihyperlipidemics and 30-Day Notice to Prior Authorize Nexletol® (Bempedoic Acid) and Nexlizet™ (Bempedoic Acid/Ezetimibe) – Appendix D Annual Review of Glaucoma Medications and 30-Day Notice to Prior Authorize Durysta™ (Bimatoprost Implant) – Appendix E Annual Review of Antiviral Medications – Appendix F Annual Review of Korlym® (Mifepristone) – Appendix G Annual Review of Turalio® (Pexidartinib) – Appendix H Annual Review of Inrebic® (Fedratinib) and Elzonris® (Tagraxofusp-erzs) – Appendix I 30-Day Notice to Prior Authorize Imcivree™ (Setmelanotide) – Appendix J U.S. Food and Drug Administration (FDA) and Drug Enforcement Administration (DEA) Updates – Appendix K Future Business ORI-4403 • P.O. BOX 26901 • OKLAHOMA CITY, OKLAHOMA 73126-0901 • (405) 271-9039 • FAX: (405) 271-2615 Oklahoma Health Care Authority Drug Utilization Review Board (DUR Board) Packet – January 13, 2021 No live January meeting. -

WO 2020/0951S3 A1 14 May 2020 (14.05.2020) I 4 P Cl I P C T
(12) INTERNATIONAL APPLICATION PI;BLISHED I. NDER THE PATENT COOPERATION TREATY (PCT) (19) World intellectual Propertv Organraation llIIlIIlllIlIlllIlIllIllIllIIIIIIIIIIIIIIIIIIIIIIllIlIlIlIllIlllIIllIlIIllllIIIIIIIIIIIIIIIIIII International Bureau (10) International Publication Number (43) International Publication Date WO 2020/0951S3 A1 14 May 2020 (14.05.2020) I 4 P Cl I P C T (51) International Patent Classification: MC. MK, MT. M., NO, PL, PT, RO, RS. SE. SI. SK. SM, A6JK3J/4166 (2006.01) A6/K&/5/t)6 (200G 01) TR). OAPI (BF, BJ, CF, CG, CI. CYI. GA, GN, GQ. GW. AGJK39/00(2006 01) C07K/6/28 (2006 01) KM,:vIL. MR, NE. SN, TD, TG) A6JP35/00(200G 01) AGJK38/68 (2019 Ol) Published: (21) International Application tiumber: »iili nrternatinrral seair 6 repurt 14rt 2113&i PCT/IB2019/059459 m Alar/ and &ilnte, the mternat»rrral app/icramn as fihrl (22) International Filing Date: crmiamerl en/«i rrr grevscale a&irl is rivrnhihiefur d«ii nlrrarl 04 November 2019 (04,11.2019) /rum P) TES"/;ST'OPE (25) Filing Langurrge: Enghsh (26) Public&stion Language: Enghsh (30) Priority Data: (&2/755.944 05 Not en&ber 2018105.11 20)8) US (i2/882.424 02 Auknrst 2019 (02 08.2019) US (71) Applicants: PFIZER INC. [US/L S], 233 East 42nd Street. Neve York. Ncw York 10017 (US). MERCK PATENT GMBH [DE/DE], Frankfurter Strasse 250, G4293 Dmm- stadi (DE) NEKTAR THERAPEUTICS [US/US]. 435 Nlission Ba) Boulmard South. Suite 100. San Francis- co, California 94158 (US). ASTELLAS PHARMA INC. f JP/JP], 2-5-1. Nihonbashi-Honcho, Chuo-Ku, Tokv o (JP) (72) Inventors: BOSHOFF, Christoffel Hendrik; c/o PFIZER INC, 235 East 42nd Street Neu York. -

Fertility & Sterility®
POSTER SESSION: LATE-BREAKING ABSTRACTS P-931 3:30 PM Wednesday, October 21, 2020 LINZAGOLIX MAY ADDRESS THE LONG-TERM P-930 3:30 PM Wednesday, October 21, 2020 TREATMENT NEEDS OF WOMEN WITH UTERINE FI- BROIDS (UF) WHO HAVE CONTRAINDICATIONS TO EFFICACYAND SAFETY OF LINZAGOLIX (LGX) FOR HORMONAL ADD-BACK THERAPY (ABT): RESULTS THE TREATMENT OF HEAVY MENSTRUAL FROM TWO PHASE 3 RANDOMIZED CLINICAL BLEEDING (HMB) DUE TO UTERINE FIBROIDS TRIALS. Linda D. Bradley, MD,1 Erica E. Marsh, MD, MSCI, FACOG,2 (UF): RESULTS FROM TWO PHASE 3 RANDOMIZED Elizabeth Garner, MD, MPH.3 1Cleveland Clinic Cleveland, OH; 2University CLINICAL TRIALS. Elizabeth A. Stewart, MD,1 Hugh S. Taylor, of Michigan, Ann Arbor, MI; 3ObsEva Inc, Boston, MA. MD,2 Robert N. Taylor, MD, PhD,3 Jacques Donnez, PhD, MD,4 Elke Bestel, MD,5 Jean-Pierre Gotteland, PhD,5 Andrew Humberstone, OBJECTIVE: GnRH antagonists comprise a new class of orally active med- PhD,5 Elizabeth Garner, MD, MPH.6 1Mayo Clinic Department of OB/ ications that dose-dependently reduce estradiol (E2) levels, allowing partial sup- GYN Reproductive Endocrinology and Infertility, Rochester, MN; 2Yale pression for symptom relief of estrogen-driven conditions without the bone University School of Medicine, New Haven, CT; 3University of Utah Health, (BMD) loss seen with full suppression. A GnRH antagonist developed using a Salt Lake City, UT; 4Polyclinique Urbain V (ELSAN Group), Brussels, full suppression dose that requires use of hormonal ABT was recently approved Belgium; 5ObsEva SA, Plan-les-Ouates, Switzerland; 6ObsEva Inc, Boston, for the treatment of UF. Its labeling lists obesity, hypertension, and dyslipidemia MA. -

UNITED STATES SECURITIES and EXCHANGE COMMISSION FORM 10-K Myovant Sciences Ltd
Table of Contents UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K (Mark One) ý ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended March 31, 2018 or o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number 001-37929 Myovant Sciences Ltd. (Exact name of registrant as specified in its charter) Bermuda 98-1343578 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) Suite 1, 3rd Floor 11-12 St. James’s Square London SW1Y 4LB United Kingdom Not Applicable (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: +44 203 318 9709 Securities registered pursuant to Section 12(b) of the Act: Title of each Class Name of each exchange on which registered Common Shares, $0.000017727 par value per share New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark whether the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý Indicate by check mark whether the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes o No ý Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Company Corporate Presentation
Focused on unmet needs in women’s reproductive health August 2021 Disclaimer Matters discussed in this presentation may constitute forward-looking statements. We expressly disclaim any obligation to update or revise the information herein, The forward-looking statements contained in this presentation reflect our views as of including the forward-looking statements, except as required by law. Please also the date of this presentation about future events and are subject to risks, note that this presentation does not constitute an offer to sell or a solicitation of an uncertainties, assumptions, and changes in circumstances that may cause our actual offer to buy any securities. results, performance, or achievements to differ significantly from those expressed or This presentation concerns products that are under clinical investigation and which implied in any forward-looking statement. Although we believe that the expectations have not yet been approved for marketing by the US Food and Drug Administration. reflected in the forward-looking statements are reasonable, we cannot guarantee It is currently limited by federal law to investigational use, and no representation is future events, results, performance, or achievements. Some of the key factors that made as to its safety or effectiveness for the purposes for which it is being could cause actual results to differ from our expectations include our plans for clinical investigated. The trademarks included herein are the property of the owners thereof development and commercialization of our product candidates; our planned clinical and are used for reference purposes only. Such use should not be construed as an trials and preclinical studies for our product candidates, including uncertainties endorsement of such products.