Company Corporate Presentation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Personalized ADT
Personalized ADT Thomas Keane MD Conflicts • Ferring • Tolemar • Bayer • Astellas • myriad Personalized ADT for the Specific Paent • Cardiac • OBesity and testosterone • Fsh • High volume metastac disease • Docetaxol • Significant LUTS Cardiovascular risk profile and ADT Is there a difference? Degarelix Belongs to a class of synthe@c drug, GnRH antagonist (Blocker) GnRH pGlu His Trp Ser Tyr Gly Leu Arg Pro Gly NH2 Leuprolide D-Leu NEt Goserelin D-Ser NH2 LHRH agonists Triptorelin D-Trp NH2 Buserelin D-Ser NEt Degarelix D-NaI D-Cpa D-PaI Aph D-Aph D-Ala NH2 N-Me ABarelix D-NaI D-Cpa D-PaI D-Asn Lys D-Ala NH2 Tyr GnRH antagonists Cetrorelix D-NaI D-Cpa D-PaI D-Cit D-Ala NH2 Ganirelix D-NaI D-CPa D-PaI D-hArg D-hArg D-Ala NH2 Millar RP, et al. Endocr Rev 2004;25:235–75 Most acute CVD events are caused By rupture of a vulnerable atherosclero@c plaque The vulnerable plaque – thin cap with inflammaon Inflammation Plaque instability is at the heart of cardiovascular disease Stable plaque Vulnerable plaque Lumen Lumen Lipid core Lipid core FiBrous cap FiBrous cap Thick Cap Thin Rich in SMC and matrix Composion Rich in inflammatory cells: proteoly@c ac@vity Poor Lipid Rich Inflammatory Inflammatory state Highly inflammatory LiBBy P. Circulaon 1995;91:2844-2850 Incidence of Both prostate cancer and CV events is highest in older men Prostate cancer CV events 3500 3500 Prostate cancer All CV disease Major CV events 3000 3000 2827.1 2500 2500 2338.9 2000 2000 1719.7 1500 1500 1152.6 1008.7 1038.7 1000 1000 641.2 545.2 571.1 Age-specific incidence per 100,000 person-years 500 500 246.9 133.7 4.3 0 0 40-49 50-59 60-69 70-79 80-89 90-99 40-49 50-59 60-69 70-79 80-89 90-99 CV, cardiovascular Major CV events = myocardial infarc@on, stroke, or death due to CV disease All CV disease = major CV events + self-reported angina or revascularisaon procedures Driver, et al. -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances" WHO/EMP/RHT/TSN/2018.1
INN Working Document 19.450 04/02/2019 Addendum1 to "The use of stems in the selection of International Nonproprietary names (INN) for pharmaceutical substances" WHO/EMP/RHT/TSN/2018.1 Programme on International Nonproprietary Names (INN) Technologies Standards and Norms (TSN) Regulation of Medicines and other health technologies (RHT) World Health Organization, Geneva © World Health Organization 2019 - All rights reserved. The contents of this document may not be reviewed, abstracted, quoted, referenced, reproduced, transmitted, distributed, translated or adapted, in part or in whole, in any form or by any means, without explicit prior authorization of the WHO INN Programme. This document contains the collective views of the INN Expert Group and does not necessarily represent the decisions or the stated policy of the World Health Organization. Addendum1 to "The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances" - WHO/EMP/RHT/TSN/2018.1 1 This addendum is a cumulative list of all new stems selected by the INN Expert Group since the publication of "The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances" 2018. ------------------------------------------------------------------------------------------------------------ -calcet/-calcet- calcium-sensing receptors (CaSR) agonists cinacalcet (88), etelcalcetide (112), evocalcet (113), tecalcet (87), upacicalcet (118) ------------------------------------------------------------------------------------------------------------ -

Degarelix for Treating Advanced Hormone- Dependent Prostate Cancer
CONFIDENTIAL UNTIL PUBLISHED NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Final appraisal determination Degarelix for treating advanced hormone- dependent prostate cancer This guidance was developed using the single technology appraisal (STA) process 1 Guidance 1.1 Degarelix is recommended as an option for treating advanced hormone-dependent prostate cancer, only in adults with spinal metastases who present with signs or symptoms of spinal cord compression. 1.2 People currently receiving treatment initiated within the NHS with degarelix that is not recommended for them by NICE in this guidance should be able to continue treatment until they and their NHS clinician consider it appropriate to stop. 2 The technology 2.1 Degarelix (Firmagon, Ferring Pharmaceuticals) is a selective gonadotrophin-releasing hormone antagonist that reduces the release of gonadotrophins by the pituitary, which in turn reduces the secretion of testosterone by the testes. Gonadotrophin- releasing hormone is also known as luteinising hormone-releasing hormone (LHRH). Because gonadotrophin-releasing hormone antagonists do not produce a rise in hormone levels at the start of treatment, there is no initial testosterone surge or tumour stimulation, and therefore no potential for symptomatic flares. National Institute for Health and Care Excellence Page 1 of 71 Final appraisal determination – Degarelix for treating advanced hormone-dependent prostate cancer Issue date: April 2014 CONFIDENTIAL UNTIL PUBLISHED Degarelix has a UK marketing authorisation for the ‘treatment of adult male patients with advanced hormone-dependent prostate cancer’. It is administered as a subcutaneous injection. 2.2 The most common adverse reactions with degarelix are related to the effects of testosterone suppression, including hot flushes and weight increase, or injection site reactions (such as pain and erythema). -

Myovant Sciences Ltd. 10K 2021 V1
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended March 31, 2021 or ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from _______ to _______ Commission file number 001-37929 Myovant Sciences Ltd. (Exact name of registrant as specified in its charter) Bermuda 98-1343578 (State or other jurisdiction of incorporation or organization) (I.R.S. Employer Identification No.) Suite 1, 3rd Floor 11-12 St. James’s Square London SW1Y 4LB United Kingdom Not Applicable (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: +44 (207) 400 3351 Securities registered pursuant to Section 12(b) of the Act: Title of each Class Trading Symbol Name of each exchange on which registered Common Shares, $0.000017727 par value per share MYOV New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Degarelix Acetate (Firmagon) Reference Number: PA.CP.PHAR.170 Effective Date: 01/18 Last Review Date: 10/18 Revision Log
Clinical Policy: Degarelix Acetate (Firmagon) Reference Number: PA.CP.PHAR.170 Effective Date: 01/18 Last Review Date: 10/18 Revision Log Description The intent of the criteria is to ensure that patients follow selection elements established by Pennsylvania Health and Wellness® clinical policy for the use of Degarelix Acetate (Firmagon®). FDA Approved Indication(s) Firmagon is indicated for treatment of advanced prostate cancer. Policy/Criteria It is the policy of Pennsylvania Health and Wellness that Firmagon is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Prostate Cancer (must meet all): 1. Diagnosis of prostate cancer; 2. Prescribed by or in consultation with an oncologist; 3. Request meets one of the following (a, b or c): a. Starting dose does not exceed 240 mg given as two injections of 120 mg each; b. Maintenance dose does not exceed 80 mg as a single injection per 28 days; c. Dose is supported by practice guidelines or peer-reviewed literature for the relevant off- label use (prescriber must submit supporting evidence). Approval duration: 12 months B. Other diagnoses/indications: Refer to PA.CP.PMN.53 1. The following NCCN recommended uses for Firmagon, meeting NCCN categories 1, 2a, or 2b, are approved per the PA.CP.PMN.53: II. Continued Approval A. Prostate Cancer (must meet all): 1. Currently receiving medication via Pennsylvania Health and Wellness benefit or member has previously met approval criteria or the Continuity of Care policy (PA.LTSS.PHAR.01) applies; 2. Member is responding positively to therapy; 3. If request is for a dose increase, request meets one of the following: a. -

Obseva SA Reports Consistent Long-Term Findings from Phase 2B EDELWEISS Trial of Linzagolix for Endometriosis-Associated Pain
ObsEva SA Reports Consistent Long-Term Findings from Phase 2b EDELWEISS trial of Linzagolix for Endometriosis-Associated Pain Geneva, Switzerland and Boston, MA – May 3, 2019 – ObsEva SA (NASDAQ: OBSV / SIX: OBSN), a clinical- stage biopharmaceutical company focused on the development and commercialization of novel therapeutics for serious conditions that compromise a woman’s reproductive health and pregnancy, today reported follow-up results from the Phase 2b EDELWEISS clinical trial of its oral gonadotropin releasing hormone (GnRH) receptor antagonist, linzagolix, for the treatment of endometriosis-associated pelvic pain. These new data include 28-week extension study treatment (52 weeks of continuous treatment), as well as the 24-week post treatment follow-up (PTFU) results for patients who did not enter the extension study after completing the initial 24-week treatment period. "We are pleased to report long-term data from the EDELWEISS trial of linzagolix, which show that in patients treated with linzagolix for 52 weeks, pelvic pain response rates are maintained with the 75mg or the 200mg dose. Bone mineral density remains within safe limits. Patients that were followed for 6 months after treatment completion continue to experience pain control, and showed BMD increase,” said Ernest Loumaye, Co-Founder and Chief Executive Officer of ObsEva. “These data further support the long term therapeutic potential of linzagolix and support the currently starting Phase 3 program for the endometriosis indication, as we anticipate initial Phase 3 clinical results later this year from the trial in uterine fibroids.” Overall Pelvic Pain reduction sustained long-term After 52 weeks of treatment, responder rates for Overall Pelvic Pain (OPP)— defined as the proportion of patients experiencing an OPP score reduction >30% from baseline using a verbal rating scale— were 69% for the 75mg once daily dose and 82% for the 200mg/100mg once daily dose. -

Follicular and Endocrine Profiles Associated with Different Gnrh
Reproductive BioMedicine Online (2012) 24, 153– 162 www.sciencedirect.com www.rbmonline.com ARTICLE Follicular and endocrine profiles associated with different GnRH-antagonist regimens: a randomized controlled trial Juan Antonio Garcı´a-Velasco a, Sanja Kupesic b, Antonio Pellicer c, Claire Bourgain d, Carlos Simo´n e, Milan Mrazek f, Paul Devroey g, Joan-Carles Arce h,* a IVI Foundation, Reproductive Endocrinology, Madrid, Spain; b Texas Tech University Health Sciences Center, Department of Obstetrics and Gynecology, El Paso, TX, USA; c IVI Foundation, Reproductive Endocrinology, Valencia, Spain; d UZ Brussel, Department of Pathology, Brussels, Belgium; e IVI Foundation, Research Department, Valencia, Spain; f ISCARE IVF a.s., Lighthouse, Prague, Czech Republic; g UZ Brussel, Centre for Reproductive Medicine, Brussels, Belgium; h Reproductive Health, Global Clinical and Non-Clinical R & D, Ferring Pharmaceuticals, Copenhagen, Denmark * Corresponding author. E-mail address: [email protected] (J.-C. Arce). Dr Juan Garcı´a-Velasco did his training in obstetrics and gynaecology at Madrid Autonoma University, and then his reproductive endocrinology and infertility fellowship at Yale University, USA (1997–1998). He is now Director of IVI-Madrid and Associate Professor at Rey Juan Carlos University. He has published over 100 peer- reviewed articles. He is an ad-hoc reviewer for the main journals in the field and serves on the editorial board of Reproductive BioMedicine Online. His main research interests are endometriosis, ovarian hyperstimulation syndrome, low responders and human implantation. Abstract This trial assessed the impact of early initiation of gonadotrophin-releasing hormone (GnRH) antagonist on follicular and endocrine profiles compared with the fixed GnRH-antagonist protocol. -

Gnrh Antagonists Have Direct Inhibitory Effects on Castration-Resistant Prostate
Author Manuscript Published OnlineFirst on July 24, 2019; DOI: 10.1158/1535-7163.MCT-18-1337 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. GnRH antagonists have direct inhibitory effects on castration-resistant prostate cancer via intracrine androgen and AR-V7 expression. Vito Cucchiara1*, Joy C. Yang1*, Chengfei Liu1, Hans H. Adomat2, Emma S. Tomlinson Guns2, Martin E. Gleave2, Allen C. Gao1,3, Christopher P. Evans1,3 1Department of Urologic Surgery, University of California at Davis, Sacramento, California. 2Vancouver Prostate Centre, University of British Columbia, Vancouver, BC, Canada 3UC Davis Comprehensive Cancer Center, University of California Davis, California. Running title: GnRH antagonist inhibits intracrine androgen and AR-V7 Keywords: Prostate Cancer, ADT, GnRH antagonist, intracrine androgen Financial Support: This work is supported in part by grants DOD PC150040P1 and Ferring Pharmaceuticals to CP Evans. Conflicts of Interest: Research support from Ferring to CPE and JCY. All other authors have no conflicts of interest. Corresponding Author: Christopher P. Evans, MD, FACS Professor and Chairman, Department of Urology Urologic Surgical Oncology University of California, Davis, School of Medicine 4860 Y St., Suite 3500 Sacramento, CA 95817 academic office tel # (916)734-7520 academic office fax # (916)734-8094 email: [email protected] *These authors contributed equally to this work Downloaded from mct.aacrjournals.org on October 6, 2021. © 2019 American Association for Cancer Research. Author Manuscript Published OnlineFirst on July 24, 2019; DOI: 10.1158/1535-7163.MCT-18-1337 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. -

EC313-A Tissue Selective SPRM Reduces the Growth and Proliferation of Uterine Fbroids in a Human Uterine Fbroid Tissue Xenograft Model Hareesh B
www.nature.com/scientificreports OPEN EC313-a tissue selective SPRM reduces the growth and proliferation of uterine fbroids in a human uterine fbroid tissue xenograft model Hareesh B. Nair1*, Bindu Santhamma1, Kalarickal V. Dileep2, Peter Binkley3, Kirk Acosta1, Kam Y. J. Zhang 2, Robert Schenken3 & Klaus Nickisch1 Uterine fbroids (UFs) are associated with irregular or excessive uterine bleeding, pelvic pain or pressure, or infertility. Ovarian steroid hormones support the growth and maintenance of UFs. Ulipristal acetate (UPA) a selective progesterone receptor (PR) modulator (SPRM) reduce the size of UFs, inhibit ovulation and lead to amenorrhea. Recent liver toxicity concerns with UPA, diminished enthusiasm for its use and reinstate the critical need for a safe, efcacious SPRM to treat UFs. In the current study, we evaluated the efcacy of new SPRM, EC313, for the treatment for UFs using a NOD-SCID mouse model. EC313 treatment resulted in a dose-dependent reduction in the fbroid xenograft weight (p < 0.01). Estradiol (E2) induced proliferation was blocked signifcantly in EC313-treated xenograft fbroids (p < 0.0001). Uterine weight was reduced by EC313 treatment compared to UPA treatment. ER and PR were reduced in EC313-treated groups compared to controls (p < 0.001) and UPA treatments (p < 0.01). UF specifc desmin and collagen were markedly reduced with EC313 treatment. The partial PR agonism and no signs of unopposed estrogenicity makes EC313 a candidate for the long-term treatment for UFs. Docking studies have provided a structure based explanation for the SPRM activity of EC313. Te unmet need for medical management of uterine fbroids (UFs) has led to the discovery of various novel agents in recent years. -

214621Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 214621Orig1s000 MULTI-DISCIPLINE REVIEW Summary Review Office Director Cross Discipline Team Leader Review Clinical Review Non-Clinical Review Statistical Review Clinical Pharmacology Review NDA/BLA Multi-disciplinary Review and Evaluation: NDA 214, 621 Relugolix NDA/BLA Multi-disciplinary Review and Evaluation Disclaimer: In this document, the sections labeled as “The Applicant’s Position” are completed by the Applicant, which do not necessarily reflect the positions of the FDA. Application Type NDA Application Number(s) NDA 214621 Priority or Standard Priority Submit Date(s) April 20, 2020 Received Date(s) April 20, 2020 PDUFA Goal Date December 20, 2020 Division/Office OND/CDER/OOD/DO1 Review Completion Date Established Name Relugolix (b) (4) (Proposed) Trade Name Pharmacologic Class Gonadotropin-releasing hormone (GnRH) receptor antagonist Code name TAK-385 Applicant Myovant Sciences, Inc. Formulation(s) oral tablet Dosing Regimen One time loading dose of 360 mg followed by 120 mg daily Applicant Proposed RELUGOLIX is a gonadotropin-releasing hormone Indication(s)/Population(s) (GnRH) antagonist indicated for the treatment of patients with advanced prostate cancer. Recommendation on Regular approval Regulatory Action Recommended RELUGOLIX is a gonadotropin-releasing hormone Indication(s)/Population(s) (GnRH) antagonist indicated for the treatment of (if applicable) patients with advanced prostate cancer. 1 Version date: January 2020 (ALL NDA/ BLA reviews) Disclaimer: In this document, the sections labeled as “The Applicant’s Position” are completed by the Applicant and do not necessarily reflect the positions of the FDA. Reference ID: 4719259 NDA/BLA Multi-disciplinary Review and Evaluation: NDA 214, 621 Relugolix Table of Contents Reviewers of Multi-Disciplinary Review and Evaluation .................................................. -
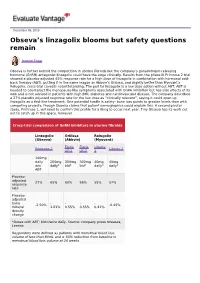
Obseva's Linzagolix Blooms but Safety Questions Remain
December 09, 2019 Obseva’s linzagolix blooms but safety questions remain Joanne Fagg Obseva is further behind the competition in uterine fibroids but the company’s gonadotropin-releasing hormone (GnRH) antagonist linzagolix could have the edge clinically. Results from the phase III Primrose 2 trial showed a placebo-adjusted 65% response rate for a high dose of linzagolix in combination with hormonal add- back therapy (ABT), putting it in the same league as Abbvie’s Orilissa, and slightly better than Myovant’s Relugolix, cross-trial caveats notwithstanding. The pull for linzagolix is a low dose option without ABT; ABT is needed to counteract the menopause-like symptoms associated with GnRH inhibition but has side effects of its own and is not advised in patients with high BMI, diabetes and cardiovascular disease. The company described a 27% placebo adjusted response rate for the low dose as “clinically relevant”, saying it could open up linzagolix as a first-line treatment. One potential hurdle is safety: bone loss points to greater levels than with competing projects, though Obseva claims that patient demographics could explain this. A second pivotal study, Primrose 1, will need to confirm this profile for a filing to occur next year. Tiny Obseva has its work cut out to catch up in this space, however. Cross-trial comparison of GnRH inhibitors in uterine fibroids Linzagolix Orilissa Relugolix (Obseva) (Abbvie) (Myovant) Elaris Elaris Liberty Primrose 2 Liberty 2 UF-1 UF-2 1 100mg daily 200mg 300mg 300mg 40mg 40mg w/o daily* bid* bid* daily* daily* ABT Placebo- adjusted 27% 65% 60% 66% 55% 57% response rate Placebo- adjusted bone - - - - -2.50% -0.45% mineral 1.81% 0.55% 0.55% 0.41% density change *Doses with ABT; bid=twice daily. -

Obseva Needs More to Beat Abbvie in Endometriosis
June 19, 2018 Obseva needs more to beat Abbvie in endometriosis Madeleine Armstrong Obseva, lagging behind its bigger rival Abbvie in the oral gonadotrophin-releasing hormone antagonist space, has a clear mission. The smaller company’s candidate, linzagolix, will need to show best-in-class efficacy if it is to have a chance of competing with Abbvie’s elagolix, which could hit the market this year in its first indication, endometriosis-associated pain. Obseva believes that it has gone some way to proving linzagolix’s superiority with data from the small phase IIb Edelweiss trial in the same disorder, and investors sent the group’s stock up 23% yesterday in response. But a look at how the results stack up against data from elagolix’s pivotal trials suggests some cause for concern (see table below). Of course, cross-trial comparisons should always be treated with caution, and this one is particularly fraught with difficulty, as the studies used different endpoints. But on the most similar measure, response on non- menstrual pain, linzagolix appears to fall short, particularly at the highest dose used, 200mg. Cross-trial comparison of linzagolix and elagolix Placebo 50mg 75mg 100mg 150mg 200mg Linzagolix/OBE2109: Edelweiss (phase IIb, NCT02778399) Response rate (primary endpoint)* 35% 49% 62% 56% - 56% P value - 0.155 0.003 0.039 - 0.034 Response rate, nonmenstrual pain 37% 46% 59% 62% - 48% P value - 0.38 0.017 0.022 - 0.297 Elagolix: Elaris EM-I, Elaris EM-II (phase III, NCT01620528, NCT01931670) Response rate, nonmenstrual pain 37% - - - 50%** 55-58%** *Response defined as reduction of ≥30% in combined menstrual and non-menstrual pelvic pain at week 12; **p<0.003; Source: Company presentations; NEJM paper.