(Notropis Topeka) Life History and Distribution in Minnesota
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fishes of South Dakota
MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 119 Fishes of South Dakota REEVE M. BAILEY AND MARVIN 0. ALLUM South Dakota State College ANN ARBOR MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN JUNE 5, 1962 MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY 01; MICHIGAN The publications of the Museum of Zoology, University of Michigan, consist of two series-the Occasional Papers and the Miscellaneous Publications. Both series were founded by Dr. Bryant Walker, Mr. Bradshaw H. Swales, and Dr. W. W. Newcomb. The Occasional Papers, publication of which was begun in 1913, serve as a medium for original studies based principally upon the collections in the Museum. They are issued separately. When a sufficient number of pages has been printed to make a volume, a title page, table of contents, and an index are supplied to libraries and indi- viduals on the mailing list for the series. The Miscellaneous Publications, which include papers on field and museum tech- niques, monographic studies, and other contributions not within the scope of the Occasional Papers, are published separately. It is not intended that they be grouped into volumes. Each number has a title page and, when necessary, a table of contents. A conlplete list of publications on Birds, Fishes, Insects, Mammals, Mollusks, and Reptiles and Amphibians is available. Address inquiries to the Director, Museum of Zoology, Ann Arbor, Michigan No. 13. Studies of the fishes of the order Cyprinodontes. By CARL L. HUBBS. (1924) 23 pp., 4 pls. ............................................. No. 15. A check-list of the fishes of the Great Lakes and tributary waters, with nomenclatorial notes and analytical keys. -

Il[Irlllii~Lliilllmlilrliilr
This document is made available electronically by the Minnesota Legislative Reference Library LEGISLATIVE REFERENCE LIBRARY LI~~~Jm'llllll~~~il[irlllii~lliilllmlilrliilas part of an ongoingr digital archiving project. http://www.leg.state.mn.us/lrl/lrl.asp 3 0307 00062 5502 MINNESOTA POLLUTION CONTROL AGENCY Division of Water Quality Municipal Section WASTEWATER DISPOSAL FACILITIES INVENTORY July 1, 1991 Summary Number Population Total State Population (1990) 4,375,099 Municipalities in the State 855 3,396,371 Municipalities with Sewer Systems 661 3,305,749 Municipalities without Sewer System 194 90,622 Municipalities having a Sewer System 2 413 without Treatment Municipalities which have only Primary 4 541 treatment (4 plants) Municipalities which have a Maximum of 502 2,684,724 Secondary Treatment (403 plants) Municipalities which have Tertiary 153 620,071 Treatment (129 plants) Municipalities having a Sewer System 659 3,305,336 with Treatment Works (536 plants) 1 Table of Contents Tables Pages 1. Municipal and Sanitary District Wastewater Treatment Works 2. Unincorporated Communities Having Sewer Systems and Wastewater Treatment Works 3. Wastewater Disposal Facilities at State Institutions 4. Wastewater Disposal Facilities at Sanatoriums and Nursing Homes 5. Wastewater Disposal Facilities at Federal Installations 6. Miscellaneous Wastewater Treatment Works 7. Facilities Operated by Sanitary Districts 8. Municipal Industrial Waste Treatment Works 9. Wastewater Disposal Facilities Operated by Indian Councils 10. Wastewater Treatment Facilities with Tertiary Treatment 11. Wastewater Treatment Facilities Completed in 1990 and 1991 12. Wastewater Treatment Facilities Under Construction 13. Municipalities Having Sewer Systems Without Treatment Works Listed Alphabetically 14. Municipalities Without Sewer Systems - Listed Alphabetically 15. -

7050.0470 CLASSIFICATIONS for SURFACE WATERS in MAJOR DRAINAGE BASINS. Subpart 1. Lake Superior Basin. the Water Use Classifica
1 REVISOR 7050.0470 7050.0470 CLASSIFICATIONS FOR SURFACE WATERS IN MAJOR DRAINAGE BASINS. Subpart 1. Lake Superior basin. The water use classifications for the listed waters in the Lake Superior basin are as identified in items A to D. See parts 7050.0425 and 7050.0430 for the classifications of waters not listed. A. Streams: (1) Ahlenius Creek, (T.53, R.14, S.9, 10): 1B, 2A, 3B; (2) Amenda Creek, (T.59, R.5, S.19, 20, 29, 30, 31; T.59, R.6, S.36): 1B, 2A, 3B; (3) Amity Creek, (T.50, R.13, S.5, 6; T.50, R.14, S.1; T.51, R.13, S.31, 32; T.51, R.14, S.26, 27, 28, 35, 36): 1B, 2A, 3B; (4) Amity Creek, East Branch (T.51, R.13, S.30, 31; T.51, R.14, S.13, 14, 15, 22, 24, 25, 36): 1B, 2A, 3B; (5) Anderson Creek, Carlton County, (T.46, R.17, S.11, 14, 15, 22, 26, 27): 1B, 2A, 3B; (6) Anderson Creek, St. Louis County, (T.49, R.15, S.16, 17, 18; T.49, R.16, S.12, 13): 1B, 2A, 3B; (7) Artichoke Creek, (T.52, R.17, S.7, 17, 18): 1B, 2A, 3B; (8) Assinika Creek, (T.63, R.1E, S.1; T.63, R.2E, S.7, 8, 16, 17, 21; T.64, R.1E, S.36; T.64, R.2E, S.31): 1B, 2A, 3B; (9) Bally Creek, (T.61, R.1W, S.3, 4, 5, 6, 7, 8, 9, 10, 11; T.61, R.2W, S.12): 1B, 2A, 3B; (10) Baptism River, East Branch, (T.57, R.6, S.6; T.57, R.7, S.1, 2, 3, 9, 10, 11, 12, 16, 17, 20; T.58, R.6, S.30, 31; T.58, R.7, S.13, 17, 19, 20, 21, 22, 23, 24, 25, 26, 29, 30, 36; T.58, R.8, S.22, 23, 24, 25, 26): 1B, 2A, 3B; (11) Baptism River, Main Branch, (T.56, R.7, S.3, 4, 5, 9, 10, 14, 15; T.57, R.7, S.20, 27, 28, 29, 33, 34): 1B, 2A, 3B; (12) Baptism River, West Branch, (T.57, R.7, S.7, 17, 18, 20; T.57, R.8, S.1, 2, 12; T.58, R.8, S.2, 3, 4, 9, 10, 11, 15, 16, 20, 21, 22, 28, 33, 34, 35, 36; T.59, R.8, S. -

Big Sioux River Watershed Strategic Plan
BIG SIOUX RIVER WATERSHED STRATEGIC PLAN In Cooperation With: South Dakota Conservation Districts South Dakota Association of Conservation Districts South Dakota Department of Environment and Natural Resources USDA Natural Resources Conservation Service Date: June 2016 Prepared by: TABLE OF CONTENTS Executive Summary ...................................................................................................... 9 1.0 Introduction ........................................................................................................ 12 1.1 Project Background and Scope .................................................................... 12 1.2 Climate ....................................................................................................... 13 1.3 Population................................................................................................... 13 1.4 Geography .................................................................................................. 16 1.5 Soils ........................................................................................................... 18 1.6 Land Use .................................................................................................... 22 1.7 Water Resources ......................................................................................... 22 1.8 Big Sioux River Watershed Improvement History ....................................... 23 1.8.1 Upper Big Sioux River Watershed ........................................................ 26 1.8.2 North-Central -

Clean Water Fund Expenditure Report
z c Clean Water Fund Expenditure Report January 2012 Legislative Charge Minn. Statutes § 114d.50, subd. 4c A state agency or other recipient of a direct appropriation from the Clean Water Fund must compile and submit all information for proposed and funded projects or programs, including the proposed measurable outcomes and all other items required under Section 3.303, subdivision 10, to the Legislative Coordinating Commission as soon as practicable or by January 15 of the applicable fiscal year, whichever comes first. Authors Estimated cost of preparing this report (as Myrna Halbach required by Minn. Stat. § 3.197) Alexis Donath Total staff time: 55 hrs. $1,388 Kurt Soular Production/duplication $63 Total $1,451 Contributors / Acknowledgements Linda Carroll The MPCA is reducing printing and mailing costs Jennifer Crea by using the Internet to distribute reports and information to wider audience. Visit our web site Editing and Graphic Design for more information. Paul Andre MPCA reports are printed on 100% post-consumer Scott Andre recycled content paper manufactured without Jerome Davis chlorine or chlorine derivatives. Beth Tegdesch Cover photo: Scott Andre Minnesota Pollution Control Agency 520 Lafayette Road North | Saint Paul, MN 55155-4194 | www.pca.state.mn.us | 651-296-6300 Toll free 800-657-3864 | TTY 651-282-5332 This report is available in alternative formats upon request, and online at www.pca.state.mn.us Document number: lrp-f-1sy12 Contents Introduction ........................................................................................................................... -

Permit Provisions and Agreement
AEDISTRIBUTION FOR PART 50 D0CET 1'A7ERTAL (T/~PO~ARy F0R4 CONTROL :O: 1722 - _____ ____ ____ ____ ENVIRO DAT Minnesota Pollution Control Agenc3 OF DOC: DATE REC'D LT MEMo Minneapolis, Minnesota 55440 3-6-73 3-14-73 Grant J.-Merritt I - .ORIG 3 OIER SENT AECi x L. Manning Muntzing SENT LOCAL __X =1; t ss:r? - 23 50-263 DESCRIPTION: ENCLOSURES: Ltr re Water Certification...with attached Regulations; permit provisions and Agreement. ACKNO G P.ANT NAbZS: Monticello DO NOT REMOVE FOR ACTION OR>ATION -n- 0 BT1LER(L) SCHWENCER(L) **ZIEMANN(L) / Co e C -IOUNGBLOOD(E) ,cWLCopies W/ CLARK(L) r/ Copies 4 Copies STOLZ(L) ROUSE(FM) REGAN(E) W/ Copies W/ Copies W/ Copies* W/ Copies GOLLER(L) VASSALLO(L) *DICKER(E) V / -Copies W/ Copies / KNIEL(L) Copies W/ Copies SCE-1EL(L) KNIGETON(E) -W/ Copies W/ Copies W/ copies W/ Copies wi Copies e~tumJ D ISTRIBUTION TECH REVIEW DENTON F & M WADE E HEND RIE GRIMES SMILEY BROWN E 0OGC, R0014 P-506A SCER )OEER GAMMILL NUSSBAUMER ...G. WILLAMS E .. *MUZINGSTAFF MACCARY KASTNER E CASE 'SHEPPARD KNIGHT(2) BALLARD. LIC ASST. GIAYBUSSO PAWLICKI SPANGLER SERVICE L IND BOYD A/T SHAO WIISON L BRAITnnk V. MOORE-L(FBR) STELLO ENVIRO GOULBOURNE L SALTZMAN DEYOUNG-L(P-w-R) HOUSTON MULLER SMITH L Ld6KOVHOLT-L NOVACK DICKER GEARIN L PIANS P. COLLINS ROSS KNIGHTON .ovDIGGS L MCDONALD IPPOLITO YOUNGBLOOD TEETS L DUBE REG OPR TEDESCO REGAN LEE L ./IE& REGION(2) LONG PROJ LEADER MAIGRET L INFO MORRIS IAINAS SHAFER F & M C. -

Wq-Rule4-12E 09/26/16 REVISOR CKM/DI RD4237
09/26/16 REVISOR CKM/DI RD4237 1.1 Pollution Control Agency 1.2 Proposed Permanent Rule Relating to Water Quality Standards and Tiered Aquatic 1.3 Life Use 1.4 7050.0140 USE CLASSIFICATIONS FOR WATERS OF THE STATE. 1.5 [For text of subps 1 and 2, see M.R.] 1.6 Subp. 3. Class 2 waters, aquatic life and recreation. Aquatic life and recreation 1.7 includes all waters of the state that support or may support fish, other aquatic life aquatic 1.8 biota, bathing, boating, or other recreational purposes and for which quality control is or 1.9 may be necessary to protect aquatic or terrestrial life or their habitats or the public health, 1.10 safety, or welfare. 1.11 [For text of subps 4 to 8, see M.R.] 1.12 7050.0150 DETERMINATION OF WATER QUALITY, BIOLOGICAL AND 1.13 PHYSICAL CONDITIONS, AND COMPLIANCE WITH STANDARDS. 1.14 [For text of subps 1 and 2, see M.R.] 1.15 Subp. 3. Narrative standards. For all Class 2 waters, the aquatic habitat, which 1.16 includes the waters of the state and stream bed, shall not be degraded in any material 1.17 manner, there shall be no material increase in undesirable slime growths or aquatic plants, 1.18 including algae, nor shall there be any significant increase in harmful pesticide or other 1.19 residues in the waters, sediments, and aquatic flora and fauna; the normal fishery and lower 1.20 aquatic biota upon which it is dependent and the use thereof shall not be seriously impaired 1.21 or endangered, the species composition shall not be altered materially, and the propagation 1.22 or migration of the fish and other aquatic biota normally present shall not be prevented or 1.23 hindered by the discharge of any sewage, industrial waste, or other wastes to the waters. -
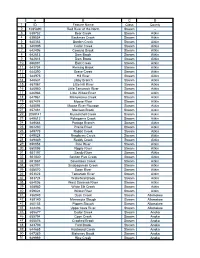
List of MN Rivers and Streams
A B C D 1 ID Feature Name Class County 2 1035890 Red River of the North Stream - 3 639752 Bear Creek Stream Aitkin 4 639854 Beckman Creek Stream Aitkin 5 640383 Borden Creek Stream Aitkin 6 640995 Cedar Creek Stream Aitkin 7 642406 Cowans Brook Stream Aitkin 8 642613 Dam Brook Stream Aitkin 9 642614 Dam Brook Stream Aitkin 10 656091 East Creek Stream Aitkin 11 643734 Fleming Brook Stream Aitkin 12 644390 Grave Creek Stream Aitkin 13 644975 Hill River Stream Aitkin 14 646631 Libby Branch Stream Aitkin 15 657067 Little Hill River Stream Aitkin 16 646950 Little Tamarack River Stream Aitkin 17 646966 Little Willow River Stream Aitkin 18 647961 Minnewawa Creek Stream Aitkin 19 657474 Moose River Stream Aitkin 20 648094 Moose River Flowage Stream Aitkin 21 657481 Morrison Brook Stream Aitkin 22 2059141 Musselshell Creek Stream Aitkin 23 649612 Pokegama Creek Stream Aitkin 24 649664 Portage Branch Stream Aitkin 25 662230 Prairie River Stream Aitkin 26 649778 Rabbit Creek Stream Aitkin 27 649828 Raspberry Creek Stream Aitkin 28 649889 Reddy Creek Stream Aitkin 29 650053 Rice River Stream Aitkin 30 650096 Ripple River Stream Aitkin 31 651197 Sandy River Stream Aitkin 32 651830 Section Five Creek Stream Aitkin 33 651867 Seventeen Creek Stream Aitkin 34 652091 Sisabagamah Creek Stream Aitkin 35 658570 Swan River Stream Aitkin 36 653023 Tamarack River Stream Aitkin 37 653724 Wakefield Brook Stream Aitkin 38 654006 West Savanna River Stream Aitkin 39 658982 White Elk Creek Stream Aitkin 40 659024 Willow River Stream Aitkin 41 456043 Duck Creek Stream -

1Iiiiiitlillnrrl~I\I~Li~I~Rlill~~~I~II11II1
This document is made available electronically by the Minnesota Legislative Reference Library 1IIIIIItlillnrrl~I\I~li~i~rlill~~~I~as part of an ongoingII11II1 digital archiving project. http://www.leg.state.mn.us/lrl/lrl.asp 3 0307 00007 4628 MINNESOTA POLLUTION CONTROL AGENCY Division of Water Quality Regulatory Compliance Section WASTEWATER DISPOSAL FACILITIES INVENTORY July 1, 1989 Summary Number Population Total State Population (1980) 4,077,148 Municipalities in the State 855 3,126,332 Municipalities with Sewer Systems 656 3,044,113 Municipalities without Sewer System 199 82,219 Municipalities having a Sewer System 2 521 without Treatment Municipalities which have only Primary 6 996 treatment (6 plants) Municipalities which have a Maximum of 499 2,449,147 Secondary Treatment (402 plants) Municipalities which have Tertiary 149 593,449 Treatment (125 plants) Municipalities having a Sewer System 654 3,043,592 with Treatment Works (533 plants) 1 ~ Table of Contents Tables Pages 1. Municipal and Sanitary District Wastewater Treatment Works 3-42 2. Unincorporated Communities Having Sewer Systems and Wastewater Treatment Works 43-44 3. Wastewater Disposal Facilities at State Institutions 45-48 4. Wastewater Disposal Facilities at Sanatoriums and Nursing Homes 49 5. Wastewater Disposal Facilities at Federal Installations 50-51 6. Miscellaneous Wastewater Treatment Works 52-57 7. Facilities Operated by Sanitary Districts 58-66 8. Municipal Industrial Waste Treatment Works 67-68 9. Wastewater Disposal Facilities Operated by Indian Councils 69 10. Wastewater Treatment Facilities with Tertiary Treatment 70-73 11. Wastewater Treatment Facilities Completed in 1988 and 1989 74-78 12. Wastewater Treatment Facilities Under Construction 79-81 13. -

The 2008 South Dakota Integrated Report for Surface Water Quality Assessment
THE 2008 SOUTH DAKOTA INTEGRATED REPORT FOR SURFACE WATER QUALITY ASSESSMENT Protecting South Dakota’s Tomorrow…Today Prepared By SOUTH DAKOTA DEPARTMENT OF ENVIRONMENT AND NATURAL RESOURCES Steven M. Pirner, Secretary SOUTH DAKOTA WATER QUALITY WATER YEARS 2002-2007 (streams) and WATER YEARS 2000-2007 (lakes) The 2008 South Dakota Integrated Report Surface Water Quality Assessment by the State of South Dakota pursuant to Sections 305(b), 303(d), and 314 of the Federal Water Pollution Control Act South Dakota Department of Environment and Natural Resources Steven M. Pirner, Secretary Table of Contents FIGURES ..................................................................................................................................................... II TABLES ......................................................................................................................................................III I. INTRODUCTION.......................................................................................................................... 1 II. EXECUTIVE SUMMARY............................................................................................................ 3 III. SURFACE WATER QUALITY ASSESSMENT........................................................................ 6 SURFACE WATER QUALITY MONITORING PROGRAM.................................................................. 6 METHODOLOGY.................................................................................................................................. -

Peak-Flow Frequency Estimates Based on Data Through Water Year 2001 for Selected Streamflow-Gaging Stations in South Dakota
Prepared in cooperation with the South Dakota Department of Transportation Peak-Flow Frequency Estimates Based on Data through Water Year 2001 for Selected Streamflow-Gaging Stations in South Dakota Scientific Investigations Report 2008–5104 U.S. Department of the Interior U.S. Geological Survey Cover photographs: Upper left: U.S. Geological Survey hydrologic technician measures the flow of the Cheyenne River near Plainview (station 06438500) during flood conditions in 1997. Photograph by U.S. Geological Survey. Upper right: James River near Redfield (station 06475000) during flood conditions in 1997. Photograph by U.S. Geological Survey. Bottom: Cars were stacked by the force of water as it pushed through Rapid City leaving the banks of Rapid Creek during the 1972 flood. Photograph courtesy of the Rapid City Journal. Peak-Flow Frequency Estimates Based on Data through Water Year 2001 for Selected Streamflow-Gaging Stations in South Dakota By Steven K. Sando, Daniel G. Driscoll, and Charles Parrett Prepared in cooperation with the South Dakota Department of Transportation Scientific Investigations Report 2008–5104 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark D. Myers, Director U.S. Geological Survey, Reston, Virginia: 2008 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1–888–ASK–USGS For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1–888–ASK–USGS Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. -

The Status and Distribution of the Topeka Shiner Notropis Topeka in Eastern South Dakota
South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange Electronic Theses and Dissertations 2001 The Status and Distribution of the Topeka Shiner Notropis topeka in Eastern South Dakota Carmen M. Blausey South Dakota State University Follow this and additional works at: https://openprairie.sdstate.edu/etd Part of the Aquaculture and Fisheries Commons, and the Natural Resources and Conservation Commons Recommended Citation Blausey, Carmen M., "The Status and Distribution of the Topeka Shiner Notropis topeka in Eastern South Dakota" (2001). Electronic Theses and Dissertations. 296. https://openprairie.sdstate.edu/etd/296 This Thesis - Open Access is brought to you for free and open access by Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. For more information, please contact [email protected]. THE STATUS AND DISTRIBUTION OF THE TOPEKA SHINER Nutropis topeka IN EASTERN SOUTH DAKOTA By Carmen M. Blausey A thesis submitted in partial fulfillment of the requirements for the l'vlastcr of Science Major in Wildlire and Fisheries Sciences (Fisheries Specialization) South Dakota State University 2001 II The Status and Distribution of the Topeka shiner Notropis topeka in Eastern South Dakota This thesis is approved as a creditable and independent investigation by a candidate for the Master of Science degree and is acceptable for meeting the thesis requirements for this degree. Acceptance of this thesis does not imply that the conclusions reached by the candidate are necessarily the conclusions of the major department.