Simulation of Highly Efficient Solar Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNIVERSITY of CALIFORNIA RIVERSIDE Fabrication And
UNIVERSITY OF CALIFORNIA RIVERSIDE Fabrication and Characterization of Organic/Inorganic Photovoltaic Devices A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Electrical Engineering by Ali Bilge Guvenc March 2012 Dissertation Committee: Dr. Mihrimah Ozkan, Chairperson Dr. Cengiz S. Ozkan Dr. Kambiz Vafai Copyright by Ali Bilge Guvenc 2012 The Dissertation of Ali Bilge Guvenc is approved: ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ Committee Chairperson University of California, Riverside To my beloved wife and daughters iv ABSTRACT OF THE DISSERTATION Fabrication and Characterization of Organic/Inorganic Photovoltaic Devices by Ali Bilge Guvenc Doctor of Philosophy, Graduate Program in Electrical Engineering University of California, Riverside, March 2012 Prof. Mihrimah Ozkan, Chairperson Energy is central to achieving the goals of sustainable development and will continue to be a primary engine for economic development. In fact, access to and consumption of energy is highly effective on the quality of life. The consumption of all energy sources have been increasing and the projections show that this will continue in the future. Unfortunately, conventional energy sources are limited and they are about to run out. Solar energy is one of the major alternative energy sources to meet the increasing demand. Photovoltaic devices are one way to harvest energy from sun and as a branch of photovoltaic devices organic bulk heterojunction photovoltaic devices have recently drawn tremendous attention because of their technological advantages for actualization of large-area and cost effective fabrication. The research in this dissertation focuses on both the mathematical modelling for better and more efficient characterization and the improvement of device power conversion efficiency. -

Optimization and Design of Photovoltaic Micro-Inverter
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2013 Optimization And Design Of Photovoltaic Micro-inverter Qian Zhang University of Central Florida Part of the Electrical and Electronics Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Zhang, Qian, "Optimization And Design Of Photovoltaic Micro-inverter" (2013). Electronic Theses and Dissertations, 2004-2019. 2811. https://stars.library.ucf.edu/etd/2811 OPTIMIZATION AND DESIGN OF PHOTOVOLTAIC MICRO-INVERTER by QIAN ZHANG B.S. Huazhong University of Science and Technology, 2006 M.S. Wuhan University, 2008 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Electrical Engineering and Computer Science in the College of Engineering and Computer Science at the University of Central Florida Orlando, Florida Summer Term 2013 Major Professor: John Shen & Issa Batarseh ©2013 Qian Zhang ii ABSTRACT To relieve energy shortage and environmental pollution issues, renewable energy, especially PV energy has developed rapidly in the last decade. The micro-inverter systems, with advantages in dedicated PV power harvest, flexible system size, simple installation, and enhanced safety characteristics are the future development trend of the PV power generation systems. The double-stage structure which can realize high efficiency with nice regulated sinusoidal waveforms is the mainstream for the micro-inverter. -

Design, Development and Fabrication of Thiophene and Benzothiadiazole Based Conjugated Polymers for Photovoltaics” Is Divided Into Five Chapters
Design, Development and Fabrication of Thiophene and Benzothiadiazole Based Conjugated Polymers for Photovoltaics A thesis submitted by Radhakrishna Ratha Roll No. 11615303 to Indian Institute of Technology Guwahati For the award of the degree of Doctor of Philosophy Center for Nanotechnology Indian Institute of Technology Guwahati Guwahati - 781039 India February, 2018 Statement INDIAN INSTITUTE OF TECHNOLOGY GUWAHATI Guwahati, Assam-781039, India Centre for Nanotechnology STATEMENT I do hereby declare that the work contained in the thesis entitled ‘Design Development and Fabrication of Thiophene and Benzothiadiazole Based Conjugated Polymers for Photovoltaics’ is the result of investigations carried out by me in the Center for Nanotechnology, Indian Institute of Technology Guwahati, Guwahati, Assam India under the supervision of Dr. Parameswar Krishnan Iyer, Professor, Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati, Assam, India. This work has not been submitted elsewhere for the award of any degree. Radhakrishna Ratha Center for Nanotechnology Indian Institute of Technology Guwahati Guwahati-781039, Assam, India February 2018 I TH-1906_11615303 Certificate INDIAN INSTITUTE OF TECHNOLOGY GUWAHATI Guwahati, Assam-781039, India Centre for Nanotechnology CERTIFICATE This is to certify that the work contained in the thesis entitled ‘Design Development and Fabrication of Thiophene and Benzothiadiazole Based Conjugated Polymers for Photovoltaics by Radhakrishna Ratha, a Ph.D. student of Center for Nanotechnology, -
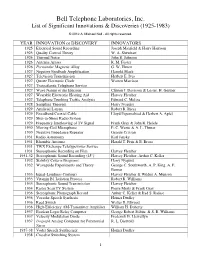
Btl Innovation List
Bell Telephone Laboratories, Inc. List of Significant Innovations & Discoveries (1925-1983) © 2012 A. Michael Noll - All rights reserved. YEAR INNOVATION or DISCOVERY INNOVATORS 1925 Electrical Sound Recording Joseph Maxfield & Harry Harrison 1925 Quality Control Theory W. A. Shewhart 1926 Thermal Noise John B. Johnson 1926 Antenna Arrays R. M. Foster 1926 Permendur Magnetic Alloy G. W. Elmen 1927 Negative Feedback Amplification Harrold Black 1927 Television Transmission Herbert E. Ives 1927 Quartz Electronic Clock Warren Marrison 1927 Transatlantic Telephone Service 1927 Wave Nature of the Electron Clinton J. Davisson & Lester. H. Germer 1927 Wearable Electronic Hearing Aid Harvey Fletcher 1927 Telephone Trunking Traffic Analysis Edward C. Molina 1928 Sampling Theorem Harry Nyquist 1929 Artificial Larynx Robert R. Riesz 1929 Broadband Coaxial Cable Lloyd Espenschied & Herbert A. Apfel 1929 Ship-to-Shore Radio System 1929 Frequency Interleaving of TV Signal Frank Gray & John R. Hefele 1930 Moving-Coil Microphone E. C. Wente & A. L. Thuras 1930 Negative Impedance Repeater George Crisson 1931 Radio Astronomy Karl Jansky 1931 Rhombic Antenna Harald T. Friis & E. Bruce 1931 TWX Exchange Teletypewriter Service 1931 Stereophonic Recording on Film Harvey Fletcher 1931-32 Stereophonic Sound Recording (45°) Harvey Fletcher, Arthur C. Keller 1932 Stability Criteria Diagrams Harry Nyquist 1932 Waveguide Experiments and Theory George C. Southworth, A. P. King, A. E. Bowen 1933 Equal-Loudness Contours Harvey Fletcher & Wilden A. Munson 1933 Vitamin B1 Isolation Process Robert R. Williams 1933 Stereophonic Sound Transmission Harvey Fletcher 1934 Raster Scan TV System Pierre Mertz & Frank Gray 1936 Stereophonc Phonograph Record Arthur C. Keller & Irad S. Rafuse 1936 Vocoder Speech Synthesis Homer Dudley 1936 Reed Switch Walter B. -

Myingyan Degree College-2017 ,Vol-8-Compressed
1 Myingyan Degree College Research Journal Vol .8, 2017 Study and Analysis on Composition Sakkarajs of Three Eigyins of Taungngu Period Tin Oo* Abstract This paper deals with the study and analysis on the composition sakkarajs (Myanmar Era) of such eigyins (classical poems addressed to a royal child extolling the glory of ancestors) as MintayarmedawEigyin, Minyekyawzwar Son Eigyin and Thakhingyi Eigyin appeared in Taungngu Period. This paper reveals the differences in the traditional specifications with regard to the composition sakkarajs of these eigyins comparing and conferring with the chronicles. Introduction The eigyingabyar (classical poem addressed to a royal child extolling the glory of ancestors; the verse begins and ends with the word ‘‘ei’’) is a kind of long ‘‘teigabyar’’(song verse or poem) soothing the royal child. It is a poem composed for the royal sons, daughters and lineages in infancy and beyond infancy. It is also a poem composing with ‘‘ei’’ k ranta or karyan (vowel sound of the letter; rhyma) at the end. The subject matters are the patriotism stimulating performances of the royal great- grandfather, royal father and royal relatives worthy of the highest praise composing eigyin. Therefore, it may be said that eigyin poem is a kind of ‘‘zartiman’’(national spirit; pride of one’s race and nation; pride of one’s lineage) literature capable of stimulating the zartithwei- zartiman (national spirit; hereditary pride and courage; patriotism). It may be designed as a kind of literature with historical value as it is like the contemporary record of that period. Throughout the history of Myanmar literature, 67 eigyins are found. -

Development of Low-Cost and High-Efficiency Commercial
DEVELOPMENT OF LOW-COST AND HIGH-EFFICIENCY COMMERCIAL SIZE N-TYPE SILICON SOLAR CELLS A Dissertation Presented to The Academic Faculty by Kyung Sun Ryu In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the School of Electrical and Computer Engineering Georgia Institute of Technology August 2015 Copyright © 2015 by Kyung Sun Ryu DEVELOPMENT OF LOW-COST AND HIGH-EFFICIENCY COMMERCIAL SIZE N-TYPE SILICON SOLAR CELLS Approved by: Dr. Ajeet Rohatgi, Advisor Dr. Gee-Kung Chang School of Electrical and Computer School of Electrical and Computer Engineering Engineering Georgia Institute of Technology Georgia Institute of Technology Dr. Thomas K. Gaylord Dr. Shreyes N. Melkote School of Electrical and Computer School of Mechanical Engineering Engineering Georgia Institute of Technology Georgia Institute of Technology Dr. Miroslav M. Begovic Department of Electrical and Computer Engineering Texas A&M University Date Approved: May 4th, 2015 This thesis work is dedicated to my parents Jae Man Ryu and Sun Ja Yeon, my brother Joong Sun Ryu, my wife Aekyung Jung, and my daughter Claire Dain Ryu. This work would have been incomplete without their encouragement, support, and love. iii ACKNOWLEDGEMENTS I would like to express my sincere gratitude to my advisor Dr. Ajeet Rohatgi for supervising my Ph.D. study and giving me the opportunity to conduct this study in the School of Electrical and Computer Engineering at Georgia Institute of Technology. I really appreciate his valuable advising and mentoring on my research. He has also spent much time in helping me to improve writing and presentation skills and to grow as a scientist. -

Nano-Composites and Their Application in Photovoltaics’
IOSR Journal of Applied Physics (IOSR-JAP) e-ISSN: 2278-4861.Volume 8, Issue 6 Ver. II (Nov. - Dec. 2016), PP 32-39 www.iosrjournals.org Review: ‘Nano-composites and their Application in Photovoltaics’ N.S.S.V.Raja Rao1, B.Nagarjun2, Y.V.P.K.Raghava3, V.Brahmajirao4 1,2,3,Dept., of Physics, G.V.P. College of Engineering (A), Visakhapatnam, India. 4 Centre for Nano Science and Technology (CNST) , G.V.P. College of Engineering(A),Visakhapatnam, India. Abstract: Current solar power technology has little chance to compete with fossil fuels or large electric grids. Today’s solar cells are simply not efficient enough and are currently too expensive for large-scale electricity generation. However, potential advances in nanotechnology may open the door to the production of cheaper and slightly more efficient solar cells. Specifically, the topics that will be discussed in this review article include nano structured inorganic materials for photovoltaic applications. Solar cells require having a large specific surface area so that sufficient dye molecules and quantum dots can be adsorbed onto the inorganic materials. These absorbed materials act as antennae for optical absorption. The most outstanding advantage of nanostructures is their ability to create architectures with significantly larger internal surface area in view of their nano-scaled size. This feature of nanostructures enables them to be suitable for use in devices such as dye- sensitized solar cells, lithium ion batteries, super capacitors, and hydrogen storage systems. This review summarizes some of the important aspects and latest developments regarding applications of nano structured materials for energy conversion and storage. -

Module-5 Unit-5 NSNT R&D on Photovoltaic Or Solar Cell
Module-5_Unit-5_NSNT R&D on Photovoltaic or Solar cell Introduction A solar or photovoltaic cell (also known as ‘solar battery’) is electrical device used to convert energy from light into electrical energy. The working of solar cell is based on photovoltaic effect, which is a physicochemical process. It is a type of photoelectric cell, which can be described as the device whose electrical properties (e.g., resistance, current, etc.) change as light is exposed onto it. Large scale photovoltaic modules are termed as solar panels, and are made up of numerous solar cells. Figure 1 A traditional solar panel made up of silicon. Electrical contacts (silver-colored strips) are printed on the wafer. Regardless of the source, whether sunlight or artificial light, solar cells are termed as photovoltaic. They can be used as photodetectors to detect light or any electromagnetic wave in the visible range, examples include infrared detectors. Besides, solar cells can also be used to measure the intensity of the light incident on them. A photovoltaic cell operates on the following three attributes: • Excitons or electron-hole pairs are generated on absorption of light. • The charge carriers are separated as electrons and holes. • The separated charge carriers are collected at their respective electrodes, i.e., electrons at the positive electrode, and hole at the negative electrode. In addition to this, a solar thermal collector can be used for direct heating or indirect generation of electrical power from the heat generated. In these devices, heat is supplied when sunlight is absorbed. Further, a photoelectrolytic or photoelectrochemical cell is ether a type of photovoltaic cell (similar to dye-sensitized solar cells developed by Edmond Becquerel) or a device which directly splits water into its constituent hydrogen and oxygen by using solar irradiation. -

INFRARED PLASTIC SOLAR CELL REVIEW Manoj Kumar1, Dharni Dhar Yadav2, Durgesh Yadav3 & Mashaba Singh4
AEGAEUM JOURNAL ISSN NO: 0776-3808 INFRARED PLASTIC SOLAR CELL REVIEW Manoj Kumar1, Dharni Dhar Yadav2, Durgesh Yadav3 & Mashaba Singh4 1Assistant Professor ABES Engineering College, Ghaziabad, UP, India. 2, 3, 4Student in Department of Mechanical Engineering ABES Engineering College, Ghaziabad, UP, India. [email protected], [email protected], [email protected], [email protected] Abstract: As we all know that electricity is very essential to us now days. It is very difficult for us to survive without electricity, with the help of electricity we are able to drive so many machines which are helpful to us but the biggest problem is that what happens if the coal is exhausted? Because most of the electricity is generated by burning of coal in power plants. Although burning of fossil fuels are the main cause of air pollution but there are many other ways to generate electricity for example: Turbines, Windmills, Solar cells etc. Our main focus is the use of solar cell, as the sun is the ultimate source of energy but we are able to consume only 1/10,000th part of sun’s energy. If we are able to consume its power more efficiently then we are able to solve so many problems of our planet without polluting the environment. Now days we are able to consume only Twenty percent of energy at most by CdTe solar panels but with the help of infrared plastic solar cell we can make it 30 percent more efficient, even the best plastic solar cells efficiency is only 6 percent. -

Ibc) Solar Cells with Screen-Printed Boron Doped Emitters
FABRICATION OF INTERDIGITATED BACK-CONTACT (IBC) SOLAR CELLS WITH SCREEN-PRINTED BORON DOPED EMITTERS A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY ATEŞCAN ALİEFENDİOĞLU IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN DEPARTMENT OF PHYSICS JANUARY 2020 Approval of the thesis: FABRICATION OF INTERDIGITATED BACK-CONTACT (IBC) SOLAR CELLS WITH SCREEN-PRINTED BORON DOPED EMITTERS submitted by ATEŞCAN ALİEFENDİOĞLU in partial fulfilment of the requirements for the degree of Master of Science in Department of Physics, Middle East Technical University by, Prof. Dr Halil Kalıpçılar Dean, Graduate School of Natural and Applied Sciences Prof. Dr Altuğ Özpineci Head of Department, Physics Prof. Dr Raşit Turan Supervisor, Department of Physics, METU Examining Committee Members: Prof. Dr Çiğdem Erçelebi Department of Physics, METU Prof. Dr Raşit Turan Department of Physics, METU Prof. Dr Mehmet Parlak Department of Physics, METU Prof. Dr H. Emrah Ünalan Dept. of Metal and Mat. Eng, METU Assist. Prof. Dr Gülsen Baytemir Department of EE Eng., Maltepe University Date: 17.01.2020 I hereby declare that all information in this document has been obtained and presented in accordance with academic rules and ethical conduct. I also declare that, as required by these rules and conduct, I have fully cited and referenced all material and results that are not original to this work. Name, Surname: Ateşcan ALİEFENDİOĞLU Signature: iv ABSTRACT FABRICATION OF INTERDIGITATED BACK-CONTACT (IBC) SOLAR CELLS WITH SCREEN-PRINTED BORON DOPED EMITTERS Aliefendioğlu, Ateşcan Master of Science, Physics Supervisor: Prof. Dr Raşit Turan January 2020, 100 Pages Interdigitated back-contact (IBC) solar cells with their unique structure, having both n and p doped regions as well all as contacts at the rear side, have the potential to achieve high-efficiency values. -
ABSTRACT Now a Day's Want of Hybrid Electrical Need Is Developing
ABSTRACT Now a day’s want of hybrid electrical need is developing within the market in view that the truth that that of use of renewable electrical give are applied on the circuit configuration as a further provide. This paper proposes an attractive inputs renewable electrical assets (RES) as a enter. Proposed community operated with the PV mobile, gasoline cell and battery and moreover average grid as a supply. So the reliability of the furnish and the complete performance of the application get increased as a quit impact. Six modes of operation are outlined for the period of the network and typical circuit is simulated within the vigor graphical persona interfacing atmosphere in MATLAB utility software utility and end result are analyzed in quick fourier transformation evaluation. CHAPTER 1 INTRODUCTION When a short-time period electrical vigor interruption happens at condominium, it's organized to be a supply of ailment. When the equal speedy-time interval interruption takes perform in an function of labor, a procedure line, or production line, the discontinue outcome may also be a costly laptop breakdown. Years inside the earlier a quick outage emerge as now not viewed a most important limitation, but this present day’s regular nation gadgets are very touchy to vigor device fluctuations. It's this vulnerability of cutting-edge-day-day science that has supplied an accelerated curiosity of electrical vigour utility variations, a fact of existence that has continuously been reward. Power strong is the time period used to explain this hassle. Energy interruptions can also be by way of intent of traditional screw usawhich incorporate hurricanes, tornadoes, and ice storms; pole-line accidents; tree contact with pressure strains and particular with ease recognized factors. -
Optimization and Design of Photovoltaic Micro-Inverter
OPTIMIZATION AND DESIGN OF PHOTOVOLTAIC MICRO-INVERTER by QIAN ZHANG B.S. Huazhong University of Science and Technology, 2006 M.S. Wuhan University, 2008 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Electrical Engineering and Computer Science in the College of Engineering and Computer Science at the University of Central Florida Orlando, Florida Summer Term 2013 Major Professor: John Shen & Issa Batarseh ©2013 Qian Zhang ii ABSTRACT To relieve energy shortage and environmental pollution issues, renewable energy, especially PV energy has developed rapidly in the last decade. The micro-inverter systems, with advantages in dedicated PV power harvest, flexible system size, simple installation, and enhanced safety characteristics are the future development trend of the PV power generation systems. The double-stage structure which can realize high efficiency with nice regulated sinusoidal waveforms is the mainstream for the micro-inverter. This thesis studied a double stage micro-inverter system. Considering the intermittent nature of PV power, a PFC was analyzed to provide additional electrical power to the system. When the solar power is less than the load required, PFC can drag power from the utility grid. In the double stage micro-inverter, the DC/DC stage was realized by a LLC converter, which could realize soft switching automatically under frequency modulation. However it has a complicated relationship between voltage gain and load. Thus conventional variable step P&O MPPT techniques for PWM converter were no longer suitable for the LLC converter. To solve this problem, a novel MPPT was proposed to track MPP efficiently.