Chapter One Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Forsterite-Anorthite-Albite System at 5 Kb Pressure Kristen Rahilly
The Forsterite-Anorthite-Albite System at 5 kb Pressure Kristen Rahilly Submitted to the Department of Geosciences of Smith College in partial fulfillment of the requirements for the degree of Bachelor of Arts John B. Brady, Honors Project Advisor Acknowledgements First I would like to thank my advisor John Brady, who patiently taught me all of the experimental techniques for this project. His dedication to advising me through this thesis and throughout my years at Smith has made me strive to be a better geologist. I would like to thank Tony Morse at the University of Massachusetts at Amherst for providing all of the feldspar samples and for his advice on this project. Thank you also to Michael Jercinovic over at UMass for his help with last-minute carbon coating. This project had a number of facets and I got assistance from many different departments at Smith. A big thank you to Greg Young and Dale Renfrow in the Center for Design and Fabrication for patiently helping me prepare and repair the materials needed for experiments. I’m also grateful to Dick Briggs and Judith Wopereis in the Biology Department for all of their help with the SEM and carbon coater. Also, the Engineering Department kindly lent their copy of LabView software for this project. I appreciated the advice from Mike Vollinger within the Geosciences Department as well as his dedication to driving my last three samples over to UMass to be carbon coated. The Smith Tomlinson Fund provided financial support. Finally, I need to thank my family for their support and encouragement as well as my friends here at Smith for keeping this year fun and for keeping me balanced. -

UNIVERSITY of CALIFORNIA RIVERSIDE Fabrication And
UNIVERSITY OF CALIFORNIA RIVERSIDE Fabrication and Characterization of Organic/Inorganic Photovoltaic Devices A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Electrical Engineering by Ali Bilge Guvenc March 2012 Dissertation Committee: Dr. Mihrimah Ozkan, Chairperson Dr. Cengiz S. Ozkan Dr. Kambiz Vafai Copyright by Ali Bilge Guvenc 2012 The Dissertation of Ali Bilge Guvenc is approved: ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ Committee Chairperson University of California, Riverside To my beloved wife and daughters iv ABSTRACT OF THE DISSERTATION Fabrication and Characterization of Organic/Inorganic Photovoltaic Devices by Ali Bilge Guvenc Doctor of Philosophy, Graduate Program in Electrical Engineering University of California, Riverside, March 2012 Prof. Mihrimah Ozkan, Chairperson Energy is central to achieving the goals of sustainable development and will continue to be a primary engine for economic development. In fact, access to and consumption of energy is highly effective on the quality of life. The consumption of all energy sources have been increasing and the projections show that this will continue in the future. Unfortunately, conventional energy sources are limited and they are about to run out. Solar energy is one of the major alternative energy sources to meet the increasing demand. Photovoltaic devices are one way to harvest energy from sun and as a branch of photovoltaic devices organic bulk heterojunction photovoltaic devices have recently drawn tremendous attention because of their technological advantages for actualization of large-area and cost effective fabrication. The research in this dissertation focuses on both the mathematical modelling for better and more efficient characterization and the improvement of device power conversion efficiency. -

Optimization and Design of Photovoltaic Micro-Inverter
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2013 Optimization And Design Of Photovoltaic Micro-inverter Qian Zhang University of Central Florida Part of the Electrical and Electronics Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Zhang, Qian, "Optimization And Design Of Photovoltaic Micro-inverter" (2013). Electronic Theses and Dissertations, 2004-2019. 2811. https://stars.library.ucf.edu/etd/2811 OPTIMIZATION AND DESIGN OF PHOTOVOLTAIC MICRO-INVERTER by QIAN ZHANG B.S. Huazhong University of Science and Technology, 2006 M.S. Wuhan University, 2008 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Electrical Engineering and Computer Science in the College of Engineering and Computer Science at the University of Central Florida Orlando, Florida Summer Term 2013 Major Professor: John Shen & Issa Batarseh ©2013 Qian Zhang ii ABSTRACT To relieve energy shortage and environmental pollution issues, renewable energy, especially PV energy has developed rapidly in the last decade. The micro-inverter systems, with advantages in dedicated PV power harvest, flexible system size, simple installation, and enhanced safety characteristics are the future development trend of the PV power generation systems. The double-stage structure which can realize high efficiency with nice regulated sinusoidal waveforms is the mainstream for the micro-inverter. -

Development of $^{100} $ Mo-Containing Scintillating
Development of 100Mo-containing scintillating bolometers for a high-sensitivity neutrinoless double-beta decay search E. Armengaud, C. Augier, A.S. Barabash, J.W. Beeman, T.B. Bekker, F. Bellini, A. Benoît, L. Bergé, T. Bergmann, J. Billard, et al. To cite this version: E. Armengaud, C. Augier, A.S. Barabash, J.W. Beeman, T.B. Bekker, et al.. Development of 100Mo-containing scintillating bolometers for a high-sensitivity neutrinoless double-beta decay search. Eur.Phys.J.C, 2017, 77 (11), pp.785. 10.1140/epjc/s10052-017-5343-2. hal-01669511 HAL Id: hal-01669511 https://hal.archives-ouvertes.fr/hal-01669511 Submitted on 11 Dec 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Eur. Phys. J. C (2017) 77:785 https://doi.org/10.1140/epjc/s10052-017-5343-2 Regular Article - Experimental Physics Development of 100Mo-containing scintillating bolometers for a high-sensitivity neutrinoless double-beta decay search E. Armengaud1, C. Augier2, A. S. Barabash3, J. W. Beeman4, T. B. Bekker5, F. Bellini6,7, A. Benoît8, L. Bergé9, T. Bergmann10, J. Billard2,R.S.Boiko11, A. Broniatowski9,12, V. Brudanin13, P. -

The Commercial Availability of Larger-Diameter Sic Substrates and Improved Crystalline Quality Has Fostered an Ever-Increasing I
THINK TANK Silicon carbide proving its value as a semiconductor substrate The commercial availability of larger-diameter SiC substrates and improved crystalline quality has fostered an ever-increasing interest in the development and manufacture of power electronic devices, exploiting the unique electrical and thermophysical properties of this wide-bandgap semiconductor material. Silicon carbide (SiC) isn’t a new material, but its use as a semiconductor substrate is helping to advance power electronics and high-frequency electronics applications. Silicon carbide is a compound semiconductor and wind energy; and industrial/commercial cost parity with silicon power devices in the material, synthesized by combining silicon applications such as server power supplies, years to come. and carbon, both from group IV of the UPS for data centers, motor drives and periodic table. It has superior properties medical imaging systems, all benefit from State-of-the-art bulk growth of SiC crystals relative to silicon, in terms of handling higher high-voltage and high-efficiency devices is carried out by the seeded sublimation voltages and temperatures. These increased made from SiC. The high-frequency devices process, often referred as the physical vapor capabilities give SiC-based chips the ability used in 5G telecommunication systems, such transport (PVT) growth method. to do tremendous work in small chip sizes. In as repeater stations, digital TV, radars and Conventional melt growth process adapted addition, SiC can switch efficiently at optoelectronic devices, also drive the for growing single crystal silicon can’t be incredible speeds. adoption of SiC material. adapted for SiC because of the lack of a stoichiometric liquid phase at reasonable Today, as markets and applications push Even though the SiC substrate cost is higher pressures. -

Growth from Melt by Micro-Pulling Down (Μ-PD) and Czochralski (Cz)
Growth from melt by micro-pulling down (µ-PD) and Czochralski (Cz) techniques and characterization of LGSO and garnet scintillator crystals Valerii Kononets To cite this version: Valerii Kononets. Growth from melt by micro-pulling down (µ-PD) and Czochralski (Cz) techniques and characterization of LGSO and garnet scintillator crystals. Theoretical and/or physical chemistry. Université Claude Bernard - Lyon I, 2014. English. NNT : 2014LYO10350. tel-01166045 HAL Id: tel-01166045 https://tel.archives-ouvertes.fr/tel-01166045 Submitted on 22 Jun 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. N° d’ordre Année 2014 THESE DE L‘UNIVERSITE DE LYON Délivrée par L’UNIVERSITE CLAUDE BERNARD LYON 1 ECOLE DOCTORALE Chimie DIPLOME DE DOCTORAT (Arrêté du 7 août 2006) Soutenue publiquement le 15 décembre 2014 par VALERII KONONETS Titre Croissance cristalline de cristaux scintillateurs de LGSO et de grenats à partir de l’état liquide par les techniques Czochralski (Cz) et micro-pulling down (μ-PD) et leurs caractérisations Directeur de thèse : M. Kheirreddine Lebbou Co-directeur de thèse : M. Oleg Sidletskiy JURY Mme Etiennette Auffray Hillemans Rapporteur M. Alain Braud Rapporteur M. Alexander Gektin Examinateur M. -

Design, Development and Fabrication of Thiophene and Benzothiadiazole Based Conjugated Polymers for Photovoltaics” Is Divided Into Five Chapters
Design, Development and Fabrication of Thiophene and Benzothiadiazole Based Conjugated Polymers for Photovoltaics A thesis submitted by Radhakrishna Ratha Roll No. 11615303 to Indian Institute of Technology Guwahati For the award of the degree of Doctor of Philosophy Center for Nanotechnology Indian Institute of Technology Guwahati Guwahati - 781039 India February, 2018 Statement INDIAN INSTITUTE OF TECHNOLOGY GUWAHATI Guwahati, Assam-781039, India Centre for Nanotechnology STATEMENT I do hereby declare that the work contained in the thesis entitled ‘Design Development and Fabrication of Thiophene and Benzothiadiazole Based Conjugated Polymers for Photovoltaics’ is the result of investigations carried out by me in the Center for Nanotechnology, Indian Institute of Technology Guwahati, Guwahati, Assam India under the supervision of Dr. Parameswar Krishnan Iyer, Professor, Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati, Assam, India. This work has not been submitted elsewhere for the award of any degree. Radhakrishna Ratha Center for Nanotechnology Indian Institute of Technology Guwahati Guwahati-781039, Assam, India February 2018 I TH-1906_11615303 Certificate INDIAN INSTITUTE OF TECHNOLOGY GUWAHATI Guwahati, Assam-781039, India Centre for Nanotechnology CERTIFICATE This is to certify that the work contained in the thesis entitled ‘Design Development and Fabrication of Thiophene and Benzothiadiazole Based Conjugated Polymers for Photovoltaics by Radhakrishna Ratha, a Ph.D. student of Center for Nanotechnology, -
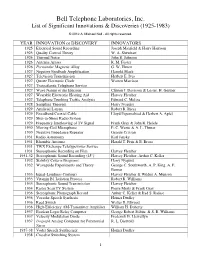
Btl Innovation List
Bell Telephone Laboratories, Inc. List of Significant Innovations & Discoveries (1925-1983) © 2012 A. Michael Noll - All rights reserved. YEAR INNOVATION or DISCOVERY INNOVATORS 1925 Electrical Sound Recording Joseph Maxfield & Harry Harrison 1925 Quality Control Theory W. A. Shewhart 1926 Thermal Noise John B. Johnson 1926 Antenna Arrays R. M. Foster 1926 Permendur Magnetic Alloy G. W. Elmen 1927 Negative Feedback Amplification Harrold Black 1927 Television Transmission Herbert E. Ives 1927 Quartz Electronic Clock Warren Marrison 1927 Transatlantic Telephone Service 1927 Wave Nature of the Electron Clinton J. Davisson & Lester. H. Germer 1927 Wearable Electronic Hearing Aid Harvey Fletcher 1927 Telephone Trunking Traffic Analysis Edward C. Molina 1928 Sampling Theorem Harry Nyquist 1929 Artificial Larynx Robert R. Riesz 1929 Broadband Coaxial Cable Lloyd Espenschied & Herbert A. Apfel 1929 Ship-to-Shore Radio System 1929 Frequency Interleaving of TV Signal Frank Gray & John R. Hefele 1930 Moving-Coil Microphone E. C. Wente & A. L. Thuras 1930 Negative Impedance Repeater George Crisson 1931 Radio Astronomy Karl Jansky 1931 Rhombic Antenna Harald T. Friis & E. Bruce 1931 TWX Exchange Teletypewriter Service 1931 Stereophonic Recording on Film Harvey Fletcher 1931-32 Stereophonic Sound Recording (45°) Harvey Fletcher, Arthur C. Keller 1932 Stability Criteria Diagrams Harry Nyquist 1932 Waveguide Experiments and Theory George C. Southworth, A. P. King, A. E. Bowen 1933 Equal-Loudness Contours Harvey Fletcher & Wilden A. Munson 1933 Vitamin B1 Isolation Process Robert R. Williams 1933 Stereophonic Sound Transmission Harvey Fletcher 1934 Raster Scan TV System Pierre Mertz & Frank Gray 1936 Stereophonc Phonograph Record Arthur C. Keller & Irad S. Rafuse 1936 Vocoder Speech Synthesis Homer Dudley 1936 Reed Switch Walter B. -

CVD Growth of Sic for High-Power and High-Frequency Applications High-Power and High- Frequency Applications
Robin Karhu Linköping Studies in Science and Technology. Dissertation No. 1973 CVD growth of SiC for CVD growth of SiC for high-power and high-frequency applications and high-frequency high-power SiC for of growth CVD high-power and high- frequency applications Robin Karhu 2019 Linköping Studies in Science and Technology Dissertation No. 1973 CVD growth of SiC for high-power and high-frequency applications Robin Karhu Semiconductor Materials Division Department of Physics, Chemistry and Biology (IFM) Linköping University Se-581 83 Linköping, Sweden Linköping 2019 Cover: Image taken with an optical microscope using a Nomarski prism for contrast. The image is taken from a 4H-SiC homoepitaxial layer grown on on-axis substrate. The image depicts a sample covered in a step-like structure. During the course of the research underlying this thesis. Robin Karhu was enrolled in Agora Materiae, a multidisciplinary doctoral program at Linköping University, Sweden. © Copyright 2019 Robin Karhu, unless otherwise noted. CVD growth of SiC for high power and high frequency applications ISBN: 978-91-7685-149-4 ISSN: 0345-7524 Linköping Studies in Science and Technology, Dissertation No. 1973. Printed in Sweden by LiU-Tryck, Linköping 2019 To my family ABSTRACT Silicon Carbide (SiC) is a wide bandgap semiconductor that has attracted a lot of interest for electronic applications due to its high thermal conductivity, high saturation electron drift velocity and high critical electric field strength. In recent years commercial SiC devices have started to make their way into high and medium voltage applications. Despite the advancements in SiC growth over the years, several issues remain. -

GEM NEWS INTERNATIONAL GEMS & GEMOLOGY SUMMER 2015 Sodic Clinopyroxene
Contributing Editors Emmanuel Fritsch, CNRS, Team 6502, Institut des Matériaux Jean Rouxel (IMN), University of Nantes, France (fritsch@cnrs- imn.fr) Kenneth Scarratt, GIA, Bangkok ([email protected]) DIAMOND Diamond-bearing eclogite xenoliths from the Ardo So Ver dykes. Specimens of gem diamond crystals in rock matrix are almost never found due to the rigorous processing that occurs during diamond mining. Occasionally, a diamond is found in a sample of the kimberlite host rock that brought it to the surface, but finding a diamond in the rock in which it likely crystallized in the earth’s mantle is ex- tremely rare. When pieces of the mantle break off and are transported to the surface in a kimberlite magma, they are called xenoliths (meaning “foreign rock,” because the man- tle rocks are not formed from the kimberlite magma itself). Recently, five small rock samples containing diamond crystals (figure 1) were sent to GIA Research for examina- tion. The samples were submitted by Charles Carmona Figure 1. These five diamond-bearing eclogite xeno- (Guild Laboratories, Los Angeles) on behalf of owners Jahn liths from the Ardo So Ver dykes in South Africa Hohne (Ekapa Mining, Kimberley, South Africa) and Vince ranged in weight from 2.24 to 12.40 grams, with the Gerardis (an occasional trader and collector of unique dia- largest measuring 2.5 centimeters in length. Photo by monds and producer of the television series “Game of Kevin Schumacher. Thrones”). The specimens were sourced over 25 years ago from a kimberlite fissure mine located 40 miles (65 km) northwest of Kimberley, South Africa. -

Myingyan Degree College-2017 ,Vol-8-Compressed
1 Myingyan Degree College Research Journal Vol .8, 2017 Study and Analysis on Composition Sakkarajs of Three Eigyins of Taungngu Period Tin Oo* Abstract This paper deals with the study and analysis on the composition sakkarajs (Myanmar Era) of such eigyins (classical poems addressed to a royal child extolling the glory of ancestors) as MintayarmedawEigyin, Minyekyawzwar Son Eigyin and Thakhingyi Eigyin appeared in Taungngu Period. This paper reveals the differences in the traditional specifications with regard to the composition sakkarajs of these eigyins comparing and conferring with the chronicles. Introduction The eigyingabyar (classical poem addressed to a royal child extolling the glory of ancestors; the verse begins and ends with the word ‘‘ei’’) is a kind of long ‘‘teigabyar’’(song verse or poem) soothing the royal child. It is a poem composed for the royal sons, daughters and lineages in infancy and beyond infancy. It is also a poem composing with ‘‘ei’’ k ranta or karyan (vowel sound of the letter; rhyma) at the end. The subject matters are the patriotism stimulating performances of the royal great- grandfather, royal father and royal relatives worthy of the highest praise composing eigyin. Therefore, it may be said that eigyin poem is a kind of ‘‘zartiman’’(national spirit; pride of one’s race and nation; pride of one’s lineage) literature capable of stimulating the zartithwei- zartiman (national spirit; hereditary pride and courage; patriotism). It may be designed as a kind of literature with historical value as it is like the contemporary record of that period. Throughout the history of Myanmar literature, 67 eigyins are found. -

First Scintillating Bolometer Tests of a CLYMENE R&D on Li2moo4
First scintillating bolometer tests of a CLYMENE R&D on Li2MoO4 scintillators towards a large-scale double-beta decay experiment G. Bu¸sea, A. Giulianib,c, P. de Marcillacb, S. Marnierosb, C. Nonesd, V. Novatib, E. Olivierib, D.V. Podab,e,∗, T. Redonb, J.-B. Sanda, P. Vebera,g, M. Vel´azqueza,f, A.S. Zolotarovad aICMCB, UMR 5026, CNRS-Universit´ede Bordeaux-INP, 33608 Pessac Cedex, France bCSNSM, Univ. Paris-Sud, CNRS/IN2P3, Universit´eParis-Saclay, 91405 Orsay, France cDISAT, Universit`adell’Insubria, 22100 Como, Italy dIRFU, CEA, Universit´eParis-Saclay, F-91191 Gif-sur-Yvette, France eInstitute for Nuclear Research, 03028 Kyiv, Ukraine fUniversit´eGrenoble Alpes, CNRS, Grenoble INP, SIMAP, 38402 Saint Martin d’H´eres, France gUniversit´eLyon, Universit´eClaude Bernard Lyon 1, CNRS, Institut Lumi´ere Mati´ere, UMR 5306, 69100 Villeurbanne, France Abstract A new R&D on lithium molybdate scintillators has begun within a project CLYMENE (Czochralski growth of Li2MoO4 crYstals for the scintillating boloMeters used in the rare EveNts sEarches). One of the main goals of the CLYMENE is a realization of a Li2MoO4 crystal growth line to be com- plementary to the one recently developed by LUMINEU in view of a mass production capacity for CUPID, a next-generation tonne-scale bolometric experiment to search for neutrinoless double-beta decay. In the present pa- per we report the investigation of performance and radiopurity of 158-g and 13.5-g scintillating bolometers based on a first large-mass (230 g) Li2MoO4 crystal scintillator developed within the CLYMENE project. In particular, a arXiv:1801.07909v2 [physics.ins-det] 8 Mar 2018 good energy resolution (2–7 keV FWHM in the energy range of 0.2–5 MeV), one of the highest light yield (0.97 keV/MeV) amongst Li2MoO4 scintillating bolometers, an efficient alpha particles discrimination (10σ) and potentially ∗Corresponding author Email address: [email protected] (D.V.