UNIVERSITY of CALIFORNIA RIVERSIDE Fabrication And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A HISTORY of the SOLAR CELL, in PATENTS Karthik Kumar, Ph.D
A HISTORY OF THE SOLAR CELL, IN PATENTS Karthik Kumar, Ph.D., Finnegan, Henderson, Farabow, Garrett & Dunner, LLP 901 New York Avenue, N.W., Washington, D.C. 20001 [email protected] Member, Artificial Intelligence & Other Emerging Technologies Committee Intellectual Property Owners Association 1501 M St. N.W., Suite 1150, Washington, D.C. 20005 [email protected] Introduction Solar cell technology has seen exponential growth over the last two decades. It has evolved from serving small-scale niche applications to being considered a mainstream energy source. For example, worldwide solar photovoltaic capacity had grown to 512 Gigawatts by the end of 2018 (representing 27% growth from 2017)1. In 1956, solar panels cost roughly $300 per watt. By 1975, that figure had dropped to just over $100 a watt. Today, a solar panel can cost as little as $0.50 a watt. Several countries are edging towards double-digit contribution to their electricity needs from solar technology, a trend that by most accounts is forecast to continue into the foreseeable future. This exponential adoption has been made possible by 180 years of continuing technological innovation in this industry. Aided by patent protection, this centuries-long technological innovation has steadily improved solar energy conversion efficiency while lowering volume production costs. That history is also littered with the names of some of the foremost scientists and engineers to walk this earth. In this article, we review that history, as captured in the patents filed contemporaneously with the technological innovation. 1 Wiki-Solar, Utility-scale solar in 2018: Still growing thanks to Australia and other later entrants, https://wiki-solar.org/library/public/190314_Utility-scale_solar_in_2018.pdf (Mar. -

Solar Cells from Selenium to Sihcon: Powering the Future. Part 2 by C M Meyer, Technical Journalist
GENERATION Solar cells from selenium to sihcon: Powering the future. Part 2 by C M Meyer, technical journalist An amazingly simple device, capable of performing efficiently nearly all the functions of an ordinary vacuum tube, was demonstrated for the first time yesterday at Bell Telephone Laboratories where it was invented. Known as the transistor, the device works on an entirely new physical principle discovered by the laboratories in the course of fundamental research into the electrical properties of solids.” Press release from Bell Telephone Laboratories announcing the first transistor, 1 July 1948 (Ref.6). The very first photoelectric cell was a pretty crude device. It was basically a large, thin layer of selenium that had been spread onto a copper metal plate, and covered with a thin, semitransparent gold-leaf film. Even by February 1953, the efficiency of such selenium cells was not very high: only a little less than 0,5%. Small wonder then that a more efficient substitute was needed. So it is hardly surprising that scientists working for Bell Telephone Laboratory, many on commercialising the newly discovered transistor, were aware of this need. One of them, Daryl Chapin, had begun work on the problem of providing small amounts of intermittent power in remote humid locations. His research originally had nothing to do with solar power, and involved wind machines, thermoelectric generators and small steam engines. At his suggestion, solar power was included in his research, almost as an afterthought. After obtaining disappointing results with a The first attempt to launch a satellite with the Vanguard rocket on 6 December 1957. -

High Energy Yield Bifacial-IBC Solar Cells Enabled by Poly-Siox Carrier Selective Passivating Contacts
High energy yield Bifacial-IBC solar cells enabled by poly-SiOx carrier selective passivating contacts Zakaria Asalieh TU Delft i High energy yield Bifacial-IBC solar cells enabled by poly-SiOX carrier selective passivating contacts By Zakaria Asalieh in partial fulfilment of the requirements for the degree of Master of Science at the Delft University of Technology, to be defended publicly on Thursday April 1, 2021 at 15:00 Supervisor: Asso. Prof. dr. Olindo Isabella Dr. Guangtao Yang Thesis committee: Assoc. Prof. dr. Olindo Isabella, TU Delft (ESE-PVMD) Prof. dr. Miro Zeman, TU Delft (ESE-PVMD) Dr. Massimo Mastrangeli, TU Delft (ECTM) Dr. Guangtao Yang, TU Delft (ESE,PVMD) ii iii Conference Abstract Evaluation and demonstration of bifacial-IBC solar cells featuring poly-Si alloy passivating contacts- Guangtao Yang, Zakaria Asalieh, Paul Procel, YiFeng Zhao, Can Han, Luana Mazzarella, Miro Zeman, Olindo Isabella – EUPVSEC 2021 iv v Acknowledgement First, I would like to express my gratitude to Dr Olindo Isabella for giving me the opportunity to work with his group. He is one of the main reasons why I chose to do my master thesis in the PVMD group. After finishing my internship on solar cells, he recommended me to join the thesis projects event where I decided to work on this interesting thesis topic. I cannot forget his support when my family and I had the Covid-19 virus. Second, I'd like to thank my daily supervisor Dr Guangtao Yang, despite supervising multiple MSc students, was able to provide me with all of the necessary guidance during this work. -

Optimization and Design of Photovoltaic Micro-Inverter
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2013 Optimization And Design Of Photovoltaic Micro-inverter Qian Zhang University of Central Florida Part of the Electrical and Electronics Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Zhang, Qian, "Optimization And Design Of Photovoltaic Micro-inverter" (2013). Electronic Theses and Dissertations, 2004-2019. 2811. https://stars.library.ucf.edu/etd/2811 OPTIMIZATION AND DESIGN OF PHOTOVOLTAIC MICRO-INVERTER by QIAN ZHANG B.S. Huazhong University of Science and Technology, 2006 M.S. Wuhan University, 2008 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Electrical Engineering and Computer Science in the College of Engineering and Computer Science at the University of Central Florida Orlando, Florida Summer Term 2013 Major Professor: John Shen & Issa Batarseh ©2013 Qian Zhang ii ABSTRACT To relieve energy shortage and environmental pollution issues, renewable energy, especially PV energy has developed rapidly in the last decade. The micro-inverter systems, with advantages in dedicated PV power harvest, flexible system size, simple installation, and enhanced safety characteristics are the future development trend of the PV power generation systems. The double-stage structure which can realize high efficiency with nice regulated sinusoidal waveforms is the mainstream for the micro-inverter. -

Building Integrated Photovoltaics in Honolulu, Hawai‘I: Assessing Urban Retrofit Applications for Power Utilization and Energy Savings
BUILDING INTEGRATED PHOTOVOLTAICS IN HONOLULU, HAWAI‘I: ASSESSING URBAN RETROFIT APPLICATIONS FOR POWER UTILIZATION AND ENERGY SAVINGS A D.ARCH PROJECT SUBMTTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAI‘I AT MĀNOA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF ARCHITECTURE MAY 2015 By Parker Lau D.Arch Project Committee: David Rockwood, Chairperson David Garmire Frank Alsup Tuan Tran Keywords: Interoperability, BIPV, Urban Retrofit, Renewable Energy, Energy Efficiency © 2015 Parker Wing Kong Lau ALL RIGHTS RESERVED ii “You never change things by fighting the existing reality. To change something, build a new model that makes the existing model obsolete.” - Buckminster Fuller iii I dedicate this dissertation to my Mother and to those I have lost along the on journey. Your love and life stands as a guiding light in times of darkness. iv Acknowledgements I would like to first thank those on my dissertation committee for the support and guidance bestowed upon me over these past two years. To my committee chair David Rockwood for taking on this subject and motivating my architectural potential. To David Garmire for entering in on the project and adding his unorthodox scientific alternatives. To Frank Alsup, who showed interest in my subject from the beginning and taught me the value of metrics in Architecture. To Tuan Tran, your patience, advice and dedication to teaching students are a valuable asset that will take you far and cement your legacy. To Jordon Little, thank you for helping me bridge the gap between theory and practice. To all the Professors and Faculty at the University of Hawai‘i at Mānoa, School of Architecture that have fostered my architectural education, I promise I will utilize it well. -

The History of Solar
Solar technology isn’t new. Its history spans from the 7th Century B.C. to today. We started out concentrating the sun’s heat with glass and mirrors to light fires. Today, we have everything from solar-powered buildings to solar- powered vehicles. Here you can learn more about the milestones in the Byron Stafford, historical development of solar technology, century by NREL / PIX10730 Byron Stafford, century, and year by year. You can also glimpse the future. NREL / PIX05370 This timeline lists the milestones in the historical development of solar technology from the 7th Century B.C. to the 1200s A.D. 7th Century B.C. Magnifying glass used to concentrate sun’s rays to make fire and to burn ants. 3rd Century B.C. Courtesy of Greeks and Romans use burning mirrors to light torches for religious purposes. New Vision Technologies, Inc./ Images ©2000 NVTech.com 2nd Century B.C. As early as 212 BC, the Greek scientist, Archimedes, used the reflective properties of bronze shields to focus sunlight and to set fire to wooden ships from the Roman Empire which were besieging Syracuse. (Although no proof of such a feat exists, the Greek navy recreated the experiment in 1973 and successfully set fire to a wooden boat at a distance of 50 meters.) 20 A.D. Chinese document use of burning mirrors to light torches for religious purposes. 1st to 4th Century A.D. The famous Roman bathhouses in the first to fourth centuries A.D. had large south facing windows to let in the sun’s warmth. -

Photovoltaic Systems Growing: an Update
International Journal of Engineering Research and Technology. ISSN 0974-3154, Volume 13, Number 9 (2020), pp. 2288-2296 © International Research Publication House. https://dx.doi.org/10.37624/IJERT/13.9.2020.2288-2296 Photovoltaic Systems Growing: An Update Ntumba Marc-Alain Mutombo Department Electrical Engineering, Mangosuthu University of Technology, Durban, KwaZulu-Natal Abstract II. PHOTOVOLTAIC CELL STRUCTURE AND ENERGY CONVERSION The photovoltaic (PV) technology as the third renewable energy (RE) generation source is growing faster than most of the RE The PV technology was born at Units States in 1954 with the technology due to intense research performed in this field. This development of the silicon PV cell made by Daryl Chapin, last year has seen an important growing of PV technology in Calvin Fuller and Gerard Pearson at Bell labs. This cell was able efficiency, cost, applications, capacity and economy. The global to convert enough SE into electricity for house appliances [2]. total solar PV installed capacity in 2018 is dominated by APAC The term PV referred to the operating mode of photodiode (China included) with 58 % of solar PV installed capacity, device in which the flow of current is entirely due to the follows by Europe (25 %), America (15 %) and MEA (2 %). transduced light energy. Based on their structure and operating mode, all PV devices are considered as some type of photodiode. Even with a decline of 16 % in 2018, the global solar PV market Fig. 1 shows the schematic block diagram of a PV cell. continue to be dominated by China with 44.4 GW installed in 2018 against 52.8 % GW in 2017. -

Design, Development and Fabrication of Thiophene and Benzothiadiazole Based Conjugated Polymers for Photovoltaics” Is Divided Into Five Chapters
Design, Development and Fabrication of Thiophene and Benzothiadiazole Based Conjugated Polymers for Photovoltaics A thesis submitted by Radhakrishna Ratha Roll No. 11615303 to Indian Institute of Technology Guwahati For the award of the degree of Doctor of Philosophy Center for Nanotechnology Indian Institute of Technology Guwahati Guwahati - 781039 India February, 2018 Statement INDIAN INSTITUTE OF TECHNOLOGY GUWAHATI Guwahati, Assam-781039, India Centre for Nanotechnology STATEMENT I do hereby declare that the work contained in the thesis entitled ‘Design Development and Fabrication of Thiophene and Benzothiadiazole Based Conjugated Polymers for Photovoltaics’ is the result of investigations carried out by me in the Center for Nanotechnology, Indian Institute of Technology Guwahati, Guwahati, Assam India under the supervision of Dr. Parameswar Krishnan Iyer, Professor, Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati, Assam, India. This work has not been submitted elsewhere for the award of any degree. Radhakrishna Ratha Center for Nanotechnology Indian Institute of Technology Guwahati Guwahati-781039, Assam, India February 2018 I TH-1906_11615303 Certificate INDIAN INSTITUTE OF TECHNOLOGY GUWAHATI Guwahati, Assam-781039, India Centre for Nanotechnology CERTIFICATE This is to certify that the work contained in the thesis entitled ‘Design Development and Fabrication of Thiophene and Benzothiadiazole Based Conjugated Polymers for Photovoltaics by Radhakrishna Ratha, a Ph.D. student of Center for Nanotechnology, -
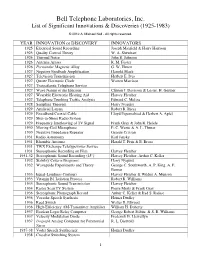
Btl Innovation List
Bell Telephone Laboratories, Inc. List of Significant Innovations & Discoveries (1925-1983) © 2012 A. Michael Noll - All rights reserved. YEAR INNOVATION or DISCOVERY INNOVATORS 1925 Electrical Sound Recording Joseph Maxfield & Harry Harrison 1925 Quality Control Theory W. A. Shewhart 1926 Thermal Noise John B. Johnson 1926 Antenna Arrays R. M. Foster 1926 Permendur Magnetic Alloy G. W. Elmen 1927 Negative Feedback Amplification Harrold Black 1927 Television Transmission Herbert E. Ives 1927 Quartz Electronic Clock Warren Marrison 1927 Transatlantic Telephone Service 1927 Wave Nature of the Electron Clinton J. Davisson & Lester. H. Germer 1927 Wearable Electronic Hearing Aid Harvey Fletcher 1927 Telephone Trunking Traffic Analysis Edward C. Molina 1928 Sampling Theorem Harry Nyquist 1929 Artificial Larynx Robert R. Riesz 1929 Broadband Coaxial Cable Lloyd Espenschied & Herbert A. Apfel 1929 Ship-to-Shore Radio System 1929 Frequency Interleaving of TV Signal Frank Gray & John R. Hefele 1930 Moving-Coil Microphone E. C. Wente & A. L. Thuras 1930 Negative Impedance Repeater George Crisson 1931 Radio Astronomy Karl Jansky 1931 Rhombic Antenna Harald T. Friis & E. Bruce 1931 TWX Exchange Teletypewriter Service 1931 Stereophonic Recording on Film Harvey Fletcher 1931-32 Stereophonic Sound Recording (45°) Harvey Fletcher, Arthur C. Keller 1932 Stability Criteria Diagrams Harry Nyquist 1932 Waveguide Experiments and Theory George C. Southworth, A. P. King, A. E. Bowen 1933 Equal-Loudness Contours Harvey Fletcher & Wilden A. Munson 1933 Vitamin B1 Isolation Process Robert R. Williams 1933 Stereophonic Sound Transmission Harvey Fletcher 1934 Raster Scan TV System Pierre Mertz & Frank Gray 1936 Stereophonc Phonograph Record Arthur C. Keller & Irad S. Rafuse 1936 Vocoder Speech Synthesis Homer Dudley 1936 Reed Switch Walter B. -

Solar Power in Building Design
Endorsements for Solar Power in Building Design Dr. Peter Gevorkian’s Solar Power in Building Design is the third book in a sequence of compre- hensive surveys in the field of modern solar energy theory and practice. The technical title does little to betray to the reader (including the lay reader) the wonderful and uniquely entertaining immersion into the world of solar energy. It is apparent to the reader, from the very first page, that the author is a master of the field and is weav- ing a story with a carefully designed plot. The author is a great storyteller and begins the book with a romantic yet rigorous historical perspective that includes the contribution of modern physics. A description of Einstein’s photoelectric effect, which forms one of the foundations of current photo- voltaic devices, sets the tone. We are then invited to witness the tense dialogue (the ac versus dc debate) between two giants in the field of electric energy, Edison and Tesla. The issues, though a century old, seem astonishingly fresh and relevant. In the smoothest possible way Dr. Gevorkian escorts us in a well-rehearsed manner through a fascinat- ing tour of the field of solar energy making stops to discuss the basic physics of the technology, manu- facturing process, and detailed system design. Occasionally there is a delightful excursion into subjects such as energy conservation, building codes, and the practical side of project implementation. All this would have been more than enough to satisfy the versed and unversed in the field of renew- able energy. -

The Silicon Solar Cell Turns 50
TELLING THE WORLD On April 25, 1954, proud Bell executives held a press conference where they impressed the media with the Bell Solar Battery powering a radio transmitter that was broadcasting voice and music. One journalist thought it important for the public to know that “linked together Daryl Chapin, Calvin Fuller, and Gerald Chapin began work in February 1952, electrically, the Bell solar cells deliver Pearson likely never imagined inventing but his initial research with selenium power from the sun at the rate of a solar cell that would revolutionize the was unsuccessful. Selenium solar cells, 50 watts per square yard, while the photovoltaics industry. There wasn’t the only type on the market, produced atomic cell announced recently by even a photovoltaics industry to revolu- too little power—a mere 5 watts per the RCA Corporation merely delivers tionize in 1952. square meter—converting less than a millionth of a watt” over the same 0.5% of the incoming sunlight into area. An article in U.S. News & World The three scientists were simply trying electricity. Word of Chapin’s problems Report speculated that one day such to solve problems within the Bell tele- came to the attention of another Bell silicon strips “may provide more phone system. Traditional dry cell researcher, Gerald Pearson. The two power than all the world’s coal, oil, batteries, which worked fine in mild scientists had been friends for years. and uranium.” The New York Times climates, degraded too rapidly in the They had attended the same university, probably best summed up what Chapin, tropics and ceased to work when needed. -

Myingyan Degree College-2017 ,Vol-8-Compressed
1 Myingyan Degree College Research Journal Vol .8, 2017 Study and Analysis on Composition Sakkarajs of Three Eigyins of Taungngu Period Tin Oo* Abstract This paper deals with the study and analysis on the composition sakkarajs (Myanmar Era) of such eigyins (classical poems addressed to a royal child extolling the glory of ancestors) as MintayarmedawEigyin, Minyekyawzwar Son Eigyin and Thakhingyi Eigyin appeared in Taungngu Period. This paper reveals the differences in the traditional specifications with regard to the composition sakkarajs of these eigyins comparing and conferring with the chronicles. Introduction The eigyingabyar (classical poem addressed to a royal child extolling the glory of ancestors; the verse begins and ends with the word ‘‘ei’’) is a kind of long ‘‘teigabyar’’(song verse or poem) soothing the royal child. It is a poem composed for the royal sons, daughters and lineages in infancy and beyond infancy. It is also a poem composing with ‘‘ei’’ k ranta or karyan (vowel sound of the letter; rhyma) at the end. The subject matters are the patriotism stimulating performances of the royal great- grandfather, royal father and royal relatives worthy of the highest praise composing eigyin. Therefore, it may be said that eigyin poem is a kind of ‘‘zartiman’’(national spirit; pride of one’s race and nation; pride of one’s lineage) literature capable of stimulating the zartithwei- zartiman (national spirit; hereditary pride and courage; patriotism). It may be designed as a kind of literature with historical value as it is like the contemporary record of that period. Throughout the history of Myanmar literature, 67 eigyins are found.