High Resolution MRI for Quantitative Assessment of Inferior Alveolar Nerve Impairment in Course of Mandible Fractures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Schwann Cell Supplementation in Neurosurgical Procedures After Neurotrauma Santiago R
ll Scienc Ce e f & o T l h a e Unda, J Cell Sci Ther 2018, 9:2 n r a r a p p u u DOI: 10.4172/2157-7013.1000281 y y o o J J Journal of Cell Science & Therapy ISSN: 2157-7013 Review Open Access Schwann Cell Supplementation in Neurosurgical Procedures after Neurotrauma Santiago R. Unda* Instituto de Biotecnología, Centro de Investigación e Innovación Tecnológica, Universidad Nacional de La Rioja, Argentina *Corresponding author: Santiago R. Unda, Instituto de Biotecnología, Centro de Investigación e Innovación Tecnológica, Universidad Nacional de La Rioja, Argentina, Tel: 3804277348; E-mail: [email protected] Rec Date: January 12, 2018, Acc Date: March 13, 2018, Pub Date: March 16, 2018 Copyright: © 2018 Santiago R. Unda. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Nerve trauma is a common cause of quality of life decline, especially in young people. Causing a high impact in personal, psychological and economic issues. The Peripheral Nerve Injury (PNI) with a several grade of axonotmesis and neurotmesis represents a real challenge for neurosurgeons. However, the basic science has greatly contribute to axonal degeneration and regeneration knowledge, making possible to implement in new protocols with molecular and cellular techniques for improve nerve re-growth and to restore motor and sensitive function. The Schwann cell transplantation from different stem cells origins is one of the potential tools for new therapies. In this briefly review is included the recent results of animal and human neurosurgery protocols of Schwann cells transplantation for nerve recovery after a PNI. -

Types and Classification of Nerve Injury: a Review
Indian Journal of Clinical Practice, Vol. 31, No. 5, October 2020 REVIEW ARTICLE Types and Classification of Nerve Injury: A Review R JAYASRI KRUPAA*, KMK MASTHAN†, ARAVINTHA BABU N‡, SONA B# ABSTRACT Nerve injuries are the most common conditions with varying symptoms, depending on the severity, intensity and nerves involved. Though much information is available on the mechanisms of injury and regeneration, reliable treatments that ensure full functional recovery are limited. The type of nerve injury alters the treatment and prognosis. This review article aims to summarize the various types of nerve injuries and their classification. Keywords: Axonotmesis, neurotmesis, neurapraxia, Wallerian degeneration erve injuries are the most common conditions bundles of fibers called fascicles, which are covered with varying symptoms depending on the by perineurium. severity, intensity and nerves involved. N Â Epineurium: Finally, groups of fascicles are bundled Recovery after any nerve injury is variable. Though together to form the peripheral nerve (such as the much information exists on the mechanisms of injury median nerve), which is covered by epineurium. and regeneration, reliable treatments that ensure full functional recovery are limited. The type of nerve CLASSIFICATION injury alters the treatment and prognosis. This review article aims to summarize the various types of nerve Classification by Type of Nerve Injury injuries and classification of nerve injuries, which is There are three types of nerve injuries: useful in understanding their pathological basis, and to evaluate the prognosis for recovery. Nerve section Understanding the basic nerve anatomy is important Nerve section can be partial or complete, sharp or for the classification and also essential to evaluate blunt. -

Enhanced Repair Effect of Toll-Like Receptor 4 Activation on Neurotmesis: Assessment Using MR Neurography
ORIGINAL RESEARCH PERIPHERAL NERVOUS SYSTEM Enhanced Repair Effect of Toll-Like Receptor 4 Activation on Neurotmesis: Assessment Using MR Neurography H.J. Li, X. Zhang, F. Zhang, X.H. Wen, L.J. Lu, and J. Shen ABSTRACT BACKGROUND AND PURPOSE: Alternative use of molecular approaches is promising for improving nerve regeneration in surgical repair of neurotmesis. The purpose of this study was to determine the role of MR imaging in assessment of the enhanced nerve regeneration with toll-like receptor 4 signaling activation in surgical repair of neurotmesis. MATERIALS AND METHODS: Forty-eight healthy rats in which the sciatic nerve was surgically transected followed by immediate surgical coaptation received intraperitoneal injection of toll-like receptor 4 agonist lipopolysaccharide (n ϭ 24, study group) or phosphate buffered saline (n ϭ 24, control group) until postoperative day 7. Sequential T2 measurements and gadofluorine M-enhanced MR imaging and sciatic functional index were obtained over an 8-week follow-up period, with histologic assessments performed at regular intervals. T2 relaxation times and gadofluorine enhancement of the distal nerve stumps were measured and compared between nerves treated with lipopolysaccharide and those treated with phosphate buffered saline. RESULTS: Nerves treated with lipopolysaccharide injection achieved better functional recovery and showed more prominent gadofluo- rine enhancement and prolonged T2 values during the degenerative phase compared with nerves treated with phosphate buffered saline. T2 values in nerves treated with lipopolysaccharide showed a more rapid return to baseline level than did gadofluorine enhancement. Histology exhibited more macrophage recruitment, faster myelin debris clearance, and more pronounced nerve regeneration in nerves treated with toll-like receptor 4 activation. -

Gvirtualxray: Open-Source Library to Simulate Transmission X-Ray Images Presented at IBFEM-4I 2018
gVirtualXRay: Open-source Library to Simulate Transmission X-ray Images Presented at IBFEM-4i 2018 Franck P. Vidal Lecturer in Visualisation School of Computer Science and Electronic Engineering Bangor University Wed 5 Sep 2018 Outline 1 Statement 2 Background 3 Simulation Model 4 Validation 5 Application examples 6 Case study 7 Conclusions 2 / 61 Parts of this work have been funded by: FP7 Career Integration Grant: Fly4PET: Fly Algorithm in PET Reconstruction for Radiotherapy Treatment Planning http://fly4pet.fpvidal.net/ FP7 ICT Collaborative project: RASimAs: Regional Anaesthesia Simulator and Assistant http://www.rasimas.eu/ The Titan Xp used for this research was donated by Thanks to Dr Jean-Michel L´etang,INSA de Lyon, for his support during the data acquisition. 3 / 61 Outline 1 Statement 2 Background 3 Simulation Model 4 Validation 5 Application examples 6 Case study 7 Conclusions 4 / 61 Rational for this Research (in Medical VR) Simulation of X-Ray attenuation extensively studied in physics; Different physically-based simulation code available; Physically-based simulation usually performed using Monte Carlo methods on CPU (often used in dosimetry for radiotherapy); Computing an image requires a very very very long time; Ray-tracing techniques are an alternative, but still relatively slow on CPU; Need for an fast open-source graphics processing unit (GPU) implementation. 5 / 61 Timeline N. Freud, \Modelling and simulation of X- and γ-ray imaging systems", PhD thesis, INSA de Lyon, France, 2003 F. P. Vidal, \Modelling the response of x-ray detectors and removing artefacts in 3D tomography", Master's thesis, INSA de Lyon, France, Sep. -
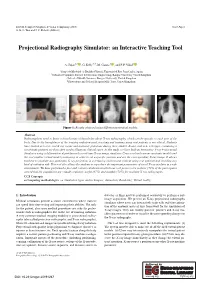
Projectional Radiography Simulator: an Interactive Teaching Tool
EG UK Computer Graphics & Visual Computing (2019) Short Paper G. K. L. Tam and J. C. Roberts (Editors) Projectional Radiography Simulator: an Interactive Teaching Tool A. Sujar1,2 , G. Kelly3,4, M. García1 , and F. P. Vidal2 1Grupo de Modelado y Realidad Virtual, Universidad Rey Juan Carlos, Spain 2School of Computer Science & Electronic Engineering, Bangor University United Kingdom 3School of Health Sciences, Bangor University, United Kingdom 4Shrewsbury and Telford Hospital NHS Trust, United Kingdom Figure 1: Results obtained using different anatomical models. Abstract Radiographers need to know a broad range of knowledge about X-ray radiography, which can be specific to each part of the body. Due to the harmfulness of the ionising radiation used, teaching and training using real patients is not ethical. Students have limited access to real X-ray rooms and anatomic phantoms during their studies. Books, and now web apps, containing a set of static pictures are then often used to illustrate clinical cases. In this study, we have built an Interactive X-ray Projectional Simulator using a deformation algorithm with a real-time X-ray image simulator. Users can load various anatomic models and the tool enables virtual model positioning in order to set a specific position and see the corresponding X-ray image. It allows teachers to simulate any particular X-ray projection in a lecturing environment without using real patients and avoiding any kind of radiation risk. This tool also allows the students to reproduce the important parameters of a real X-ray machine in a safe environment. We have performed a face and content validation in which our tool proves to be realistic (72% of the participants agreed that the simulations are visually realistic), useful (67%) and suitable (78%) for teaching X-ray radiography. -

Radiologists : Threatened by a Veritable Identity Crisis?
Current Trends in Clinical & Medical Imaging ISSN: 2573-2609 Opinion Curr Trends Clin Med Imaging Volume 2 Issue 1 - September 2017 Copyright © All rights are reserved by Werner Albert Golder DOI: 10.19080/CTCMI.2017.02.555578 Radiologists : Threatened by a Veritable Identity Crisis? Werner Albert Golder* Association D`Imagerie Medicale, France Submission: September 11, 2017 ; Published: September 18, 2017 *Corresponding author: Email: Werner Albert Golder, Md Phd, Professor Of Radiology 23, Rue De L`Oriflamme, F-84000 Avignon, France; Introduction Radiologists have always found it harder than representatives Unlike the conventional radiograph, the digital radiograph of other disciplines to ensure that their patients perceive is nolonger a definitive, unique document, this product can and recognize them as true physicians rather than medical no longer claim unrestricted copyright. Although radiologists technicians. They are too strongly and too unilaterally associated obtain the dataset, they have no influence over what is done with with the machines they operate and with their products; in many it and what is made of it. The radiographic representation they cases, they are also too far removed from the pain and anxiety have chosen is only one of many alternative ways of presenting that prompted the patients to see a physician. the dataset optically. Many radiologists have come to terms with this situation, This limitation applies both to projectional radiography and accepting their role as representatives of a paramedical science diagnostic cross-sectional imaging. Whoever receives the raw more or less uncomplainingly and trying to make the best of data of an examination with the data pack can use the integrated things. -

Perioperative Upper Extremity Peripheral Nerve Injury and Patient Positioning: What Anesthesiologists Need to Know
Anaesthesia & Critical Care Medicine Journal ISSN: 2577-4301 Perioperative Upper Extremity Peripheral Nerve Injury and Patient Positioning: What Anesthesiologists Need to Know Kamel I* and Huck E Review Article Lewis Katz School of Medicine at Temple University, USA Volume 4 Issue 3 Received Date: June 20, 2019 *Corresponding author: Ihab Kamel, Lewis Katz School of Medicine at Temple Published Date: August 01, 2019 University, MEHP 3401 N. Broad street, 3rd floor outpatient building ( Zone-B), DOI: 10.23880/accmj-16000155 Philadelphia, United States, Tel: 2158066599; Email: [email protected] Abstract Peripheral nerve injury is a rare but significant perioperative complication. Despite a variety of investigations that include observational, experimental, human cadaveric and animal studies, we have an incomplete understanding of the etiology of PPNI and the means to prevent it. In this article we reviewed current knowledge pertinent to perioperative upper extremity peripheral nerve injury and optimal intraoperative patient positioning. Keywords: Nerve Fibers; Proprioception; Perineurium; Epineurium; Endoneurium; Neurapraxia; Ulnar Neuropathy Abbreviations: PPNI: Perioperative Peripheral Nerve 2018.The most common perioperative peripheral nerve Injury; MAP: Mean Arterial Pressure; ASA CCP: American injuries involve the upper extremity with ulnar Society of Anesthesiology Closed Claims Project; SSEP: neuropathy and brachial plexus injury being the most Somato Sensory Evoked Potentials frequent [3,4]. In this article we review upper extremity PPNI with regards to anatomy and physiology, Introduction mechanisms of injury, risk factors, and prevention of upper extremity PPNI. Perioperative peripheral nerve injury (PPNI) is a rare complication with a reported incidence of 0.03-0.1% [1,2]. Anatomy and Physiology of Peripheral PPNI is a significant source of patient disability and is the Nerves second most common cause of anesthesia malpractice claims [3,4]. -

Development of a Z-Stack Projection Imaging Protocol for a Nerve Allograft
DEVELOPMENT OF A Z-STACK PROJECTION IMAGING PROTOCOL FOR A NERVE ALLOGRAFT by SELVAANISH SELVAM Submitted in partial fulfillment of the requirements for the degree of Master of Science Dissertation Advisor: Dr. George F. Muschler Department of Biomedical Engineering CASE WESTERN RESERVE UNIVERSITY August 2018 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis of Selvaanish Selvam candidate for the degree of (Master of Science) *. Committee Chair Dr. George F. Muschler Committee Member Dr. Eben Alsberg Committee Member Dr. Robert Kirsch Committee Member Cynthia Boehm Date of Defense May 4th 2018 *we also certify that written approval has been obtained For any proprietary material contained therein 2 Table of Contents List of Tables……………………………………………………………………..4 Figures List……………………………………………………………………….5 Acknowledgments………………………………………………………………..6 List of Abbreviations………………………………………………………….....7 Abstract…………………………………………………………………………...8 Introduction……………………………………………………………………..10 Methods………………………………………………………………………….21 Data and Analysis………………………………………………………………31 Conclusions and Future Directions……………………………………………51 References……………………………………………………………………….53 3 List of Tables Table 1: Overall summary of ratios and retention rates……………………..31 Table 2: Selection Ratio for grafts (3X – 10-minute rinse) ………………….35 Table 3: Selection Ratio for grafts (15-minute soak) ………………………...35 4 List of Figures: Figure 1: 2D 10X DAPI stained image using current techniques…….……….9 Figure 2: Peripheral Nerve Anatomy………………………………………….10 -

Restoration of Neurological Function Following Peripheral Nerve Trauma
International Journal of Molecular Sciences Review Restoration of Neurological Function Following Peripheral Nerve Trauma Damien P. Kuffler 1,* and Christian Foy 2 1 Institute of Neurobiology, Medical Sciences Campus, University of Puerto Rico, 201 Blvd. del Valle, San Juan, PR 00901, USA 2 Section of Orthopedic Surgery, Medical Sciences Campus, University of Puerto Rico, San Juan, PR 00901, USA; [email protected] * Correspondence: dkuffl[email protected] Received: 12 January 2020; Accepted: 3 March 2020; Published: 6 March 2020 Abstract: Following peripheral nerve trauma that damages a length of the nerve, recovery of function is generally limited. This is because no material tested for bridging nerve gaps promotes good axon regeneration across the gap under conditions associated with common nerve traumas. While many materials have been tested, sensory nerve grafts remain the clinical “gold standard” technique. This is despite the significant limitations in the conditions under which they restore function. Thus, they induce reliable and good recovery only for patients < 25 years old, when gaps are <2 cm in length, and when repairs are performed <2–3 months post trauma. Repairs performed when these values are larger result in a precipitous decrease in neurological recovery. Further, when patients have more than one parameter larger than these values, there is normally no functional recovery. Clinically, there has been little progress in developing new techniques that increase the level of functional recovery following peripheral nerve injury. This paper examines the efficacies and limitations of sensory nerve grafts and various other techniques used to induce functional neurological recovery, and how these might be improved to induce more extensive functional recovery. -

Painful Abdominal Wall Nerves
CASE REPORT Reconstructive Treatment of Painful Nerves in the Abdominal Wall Using Processed Nerve Allografts Andrew Bi, BS Eugene Park, MD Summary: Neuromas can be a debilitating cause of pain and often negatively af- Gregory A. Dumanian, MD fect patients’ quality of life. One effective method of treatment involves surgical resection of the painful neuroma and use of a processed nerve allograft to repair the injured nerve segment. Giving the nerve “somewhere to go and something to do” has been shown to effectively alleviate pain in upper and lower extremities. We present the first report of this concept to treat a painful neuroma of the ab- dominal wall that developed following a laparoscopic gastric bypass. The neuroma was excised, and the affected nerve was reconstructed using a processed nerve allograft as an interposition graft, with resolution of pain and gradual return of normal sensation. Patient-reported outcomes were measured using the Patient Reported Outcomes Measurement Information System. Neuroma excision with concurrent interposition grafting using processed nerve allografts may be a promising method of treatment for postsurgical painful neuromas of the trunk. Bi et al. (Plast Reconstr Surg Glob Open 2018;6:e1670; doi: 10.1097/GOX.0000000000001670; Published online 6 March 2018.) INTRODUCTION and unacceptable repeat surgical rates.2 And while wrap- Painful neuromas can form following any surgery when ping the distal point of a painful nerve postneuroma exci- nerves are injured due to cautery or traction. Nerve injury sion -

Patient Dose Audit in Computed Tomography at Cancer Institute of Guyana
al Diag ic no d s e t M i c f o M l e a t h n r o d u s o J ISSN: 2168-9784 Journal of Medical Diagnostic Methods Research Article Patient Dose Audit in Computed Tomography at Cancer Institute of Guyana Ramzee Small1, Petal P Surujpaul2*, and Sayan Chakraborty3 1Medical Imaging Technologist, University of Guyana, Guyana 2Medical Physics, Georgetown Public Hospital Cooperation (GPHC), University of Guyana, Guyana Abstract Objective: This study is the first investigation on computed tomography (CT) unit in Guyana at the Cancer Institute of Guyana (CIG), aimed at performing an audit on the radiation dose estimated by the GE LightSpeed QXi CT unit for common computed tomography examinations Method: A RaySafe X2 CT calibration detector were used to obtained measurements for common CT examinations (head, neck, chest, abdomen, pelvis, upper extremity and lower extremity) done in free air as the control (36 data) and also with patients (35 data). Patient’s measurement were limited to the head, chest, abdomen and pelvis CT examinations. Exposure/electro-technical parameters and dose metrics (CTDIvol, DLP) were recorded and the k- coefficient conversion factor established by National Radiology Protection Board (NRPB) were used to calculate the effective dose associated with each examination. Results: The results indicated that the CT unit overestimated the dose for patient measurements and underestimated the dose for measurements taken In-Air with the exception of the head protocol which showed overestimation with the patient measurements. Both over- and underestimation were documented for the neck protocol. Comparison of estimated dose between published data shows that there are variations in techniques and radiation dose across institution for similar examinations and that the protocols reported by CIG documented an overall higher effective dose. -

Axonotmesis of the Sciatic Nerve
Diagnostic and Interventional Imaging (2012) 93, 398—400 LETTER / Neurology Axonotmesis of the sciatic nerve a,∗ b c c M. Ohana , S. Quijano-Roy , F. Colas , C. Lebreton , c c C. Vallée , R.-Y. Carlier a Radiology Department, Nouvel Hôpital Civil, Strasbourg University Hospitals, 1, place de l’Hôpital, 67000 Strasbourg, France b Paediatrics Department, Raymond-Poincaré Hospital, 104, boulevard Raymond-Poincaré, 92380 Garches, France c Radiology Department, Raymond-Poincaré Hospital, 104, boulevard Raymond-Poincaré, 92380 Garches, France Case report KEYWORDS Peripheral nerve; We report the case of an eight-year old girl who was admitted for aftercare and rehabilita- MRI; tion one month after a serious head injury that required a four-day stay in intensive care. Axonotmesis Initial investigations did not show any evidence of post-traumatic injury. During her admission, she developed significant pain in the left buttock radiating to the lower limb associated with a sensorimotor deficit. These disabling pains persisted at rest. The clinical examination revealed that the patient had great difficulty walking, presenting a limp, a tender point on palpation of the left buttock radiating to the thigh and the leg along a posterolateral course, with hyperaesthesia in the whole area. Extension of the leg and both flexion and extension of the foot were impossible; hip flexion was normal. Hypoaesthesia was noted on the inside of the left leg and foot. The left patellar and Achilles reflexes were absent. Vital signs were normal. First-line magnetic resonance imaging (MRI) of the lumbar spine did not reveal any abnormalities. An MRI of the pelvis and lower limbs was then carried out and this highlighted involve- ment of the sciatic nerve along its whole extra-perineal course (Fig.