Induced Mutations for Food and Energy Security: Challenge of Inducing Unique Mutants for New Cultivars and Molecular Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tuesday July 30, 1996
7±30±96 Tuesday Vol. 61 No. 147 July 30, 1996 Pages 39555±39838 federal register 1 II Federal Register / Vol. 61, No. 147 / Tuesday, July 30, 1996 SUBSCRIPTIONS AND COPIES PUBLIC Subscriptions: Paper or fiche 202±512±1800 FEDERAL REGISTER Published daily, Monday through Friday, Assistance with public subscriptions 512±1806 (not published on Saturdays, Sundays, or on official holidays), by General online information 202±512±1530 the Office of the Federal Register, National Archives and Records Administration, Washington, DC 20408, under the Federal Register Single copies/back copies: Act (49 Stat. 500, as amended; 44 U.S.C. Ch. 15) and the Paper or fiche 512±1800 regulations of the Administrative Committee of the Federal Register Assistance with public single copies 512±1803 (1 CFR Ch. I). Distribution is made only by the Superintendent of Documents, U.S. Government Printing Office, Washington, DC FEDERAL AGENCIES 20402. Subscriptions: The Federal Register provides a uniform system for making Paper or fiche 523±5243 available to the public regulations and legal notices issued by Assistance with Federal agency subscriptions 523±5243 Federal agencies. These include Presidential proclamations and For other telephone numbers, see the Reader Aids section Executive Orders and Federal agency documents having general applicability and legal effect, documents required to be published at the end of this issue. by act of Congress and other Federal agency documents of public interest. Documents are on file for public inspection in the Office of the Federal Register the day before they are published, unless earlier filing is requested by the issuing agency. -

Bilevel Rail Car - Wikipedia
Bilevel rail car - Wikipedia https://en.wikipedia.org/wiki/Bilevel_rail_car Bilevel rail car The bilevel car (American English) or double-decker train (British English and Canadian English) is a type of rail car that has two levels of passenger accommodation, as opposed to one, increasing passenger capacity (in example cases of up to 57% per car).[1] In some countries such vehicles are commonly referred to as dostos, derived from the German Doppelstockwagen. The use of double-decker carriages, where feasible, can resolve capacity problems on a railway, avoiding other options which have an associated infrastructure cost such as longer trains (which require longer station Double-deck rail car operated by Agence métropolitaine de transport platforms), more trains per hour (which the signalling or safety in Montreal, Quebec, Canada. The requirements may not allow) or adding extra tracks besides the existing Lucien-L'Allier station is in the back line. ground. Bilevel trains are claimed to be more energy efficient,[2] and may have a lower operating cost per passenger.[3] A bilevel car may carry about twice as many as a normal car, without requiring double the weight to pull or material to build. However, a bilevel train may take longer to exchange passengers at each station, since more people will enter and exit from each car. The increased dwell time makes them most popular on long-distance routes which make fewer stops (and may be popular with passengers for offering a better view).[1] Bilevel cars may not be usable in countries or older railway systems with Bombardier double-deck rail cars in low loading gauges. -

Plan for Opening the Hokuriku Shinkansen Line Between Nagano and Kanazawa
East Japan Railway Company West Japan Railway Company August 27, 2014 Plan for Opening the Hokuriku Shinkansen Line between Nagano and Kanazawa East Japan Railway Company and West Japan Railway Company hereby announce that they have finalized the outline for a plan to open the Hokuriku Shinkansen Line between Nagano and Kanazawa. Details are as follows. ■Opening the Hokuriku Shinkansen Line to Kanazawa ◇Opening date : Saturday, March 14, 2015 ◇Types/Trips : ・Kagayaki (The fastest operation between Tōkyō and Kanazawa) 10 round trips/day ・Hakutaka (Stopping at most stations between Tōkyō and Kanazawa) 14 round trips/day (Stopping at most stations between Nagano and Kanazawa) 1 round trip /day ・Tsurugi (Shuttle service between Toyama and Kanazawa) 18 round trips/day ・Asama (Current Nagano Shinkansen service between Tōkyō and Nagano) 16 round trips/day T U Ō K H T A K S U N I J I U K T S K ō e m u o a n a a e a i ō t n u o h a k n i m n k n r k d g y e o a r y i n y o y a j a a u u a a a t i z o a n a ō a g ō s k i d n m s g u b m ‐ z a w a a z a o a u a k e a T a y a k h a i m w i ‐ a w a s i a w r y a o k a a e r a a ō n d u k s o a n ō e k a n a Kagayaki ● ■ ● ● ● ● ● ● ● ■ ■ ■ ■ ■ ● ■ ● ● ● ● ● ● Hakutaka ● ● ● ● ● ● ● ● Tsurugi ● ● ● Asama ● ● ● ■ ■ ● ■ ● ● ● ● ● Stopped at by all services, ■ Passed by some services ◇Configuration : Series E7 and W7: 12 car trains * Some Asama services operated with Series E2: 8 car trains ◇Fastest travel times : ・Tōkyō-Kanazawa: 2 hours 28 minutes ・Tōkyō-Toyama: 2 hours 08 minutes ■Subsequent Termination of Limited Express and Other Services on Conventional Lines ◇The following train services will be terminated subsequent to the opening of the Hokuriku Shinkansen Line to Kanazawa. -

Genotypic Performance, Adaptability and Stability in Special Types of Irrigated Rice Using Mixed Models1
Revista Ciência Agronômica, v. 50, n. 1, p. 66-75, jan-mar, 2019 Centro de Ciências Agrárias - Universidade Federal do Ceará, Fortaleza, CE Scientific Article www.ccarevista.ufc.br ISSN 1806-6690 Genotypic performance, adaptability and stability in special types of irrigated rice using mixed models1 Desempenho genotípico, adaptabilidade e estabilidade em tipos especiais de arroz irrigado via modelos mistos Eduardo Anibele Streck2*, Ariano Martins de Magalhães Júnior3, Gabriel Almeida Aguiar2, Paulo Henrique Karling Facchinello2 and Paulo Ricardo Reis Fagundes3 ABSTRACT - There is an increasing demand for special types of rice that have black or red pericarp, low amylose (subspecie japonica) or aromatic. However, intense breeding process for obtaining and indicating genetically promising new cultivars and adapted to cultivation environments are demanded. In this sense, for evaluating the special types of irrigated rice genotypes, and determining the adaptability and stability of these using mixed models. First, a preliminary agronomic evaluation of genotypes for special types of irrigated rice was performed, and later, the agronomically promising genotypes were evaluated in multi-site trials, aiming at verifying the interactions with the environment. Statistical analyzes were performed considering mixed models, using the SELEGEN-REML/BLUP software. High genotypic variability among genotypes for special types of irrigated rice was observed, obtaining some agronomically promising and with good adaptability and stability, with high accuracy and selection efficiency using mixed models. The observed wide diversity and rice genetic variability presented new prospects and opportunities for producers for acquiring food of higher added value to consumer market. Key words: Oryza sativa. Black rice. Red rice. Aromatic rice. -

The Rail Pass for Foreign Tourists Visiting Japan, JR EAST PASS and Other Passes, Are Getting Even Easier to Use!
December 17, 2020 East Japan Railway Company The Rail Pass for Foreign Tourists Visiting Japan, JR EAST PASS and Other Passes, are Getting Even Easier to Use! East Japan Railway Company (Headquarters: Shibuya-ku, Tokyo, President: Yuji Fukasawa, hereinafter "JR East") has offered a number of rail passes for various areas of Japan to meet the needs of foreign tourists visiting Japan, but there have been some inconveniences to use as the customers have to show rail passes to the station staff at the ticket office or the staff attended gate. To provide an even more seamless service, JR East will introduce a service that enables customers to use the automatic ticket gates, and purchase passes and reserve seats at the Reserved Seat Ticket Vending Machines that is compatible with all rail passes offered by JR East. Therefore, there will been some changes to the validity and price of the rail passes offered by JR East. We are striving to provide an even higher quality service to foreign tourists visiting Japan by offering a stress-free service that allows the purchase of tickets and reservation of seats without having to wait in line at the ticket counter. 1. Expanding seamless services (1) Use of automatic ticket gates for both Shinkansen and local train lines Pass holders will be able to use the automatic ticket gates within the areas that rail passes are valid. Until now Tickets sold from April 2021 Passes have to be shown to Customers will be able to use the automatic the station staff before ticket gate. -

The Bulletin 2017-06
ERA BULLETIN — JUNE, 2017 The Bulletin Electric Railroaders’ Association, Incorporated Vol. 60, No. 6 June, 2017 The Bulletin STATEN ISLAND’S 157-YEAR-OLD RAILROAD Published by the Electric Staten Island’s trains have been providing quate service with only one locomotive, it Railroaders’ Association, regular service for more than a century, but decided to buy another one. When the trains Incorporated, PO Box most people living in the other boroughs are were running less than a year, it was unable 3323, New York, New York 10163-3323. not aware that the railroad exists. to pay for the locomotives and the creditors The Staten Island Rail Road Company was threatened to seize the property. To protect incorporated on October 18, 1851 to con- the assets, the company declared bankrupt- For general inquiries, or struct a railroad from the easterly shore of cy and Cornelius Vanderbilt’s son, William, Bulletin submissions, contact us at bulletin@ Staten Island between Quarantine and Clif- was appointed receiver. erausa.org. ERA’s ton to a point nearly opposite Amboy, New Meanwhile, the company bought the pri- website is Jersey. Construction began in November, vately-owned ferries, which provided unrelia- www.erausa.org. 1855 and was completed in 1860. This 13- ble service between South Ferry (Manhattan) mile route extended from Townsend’s Dock and Staten Island. Also bought and operated Editorial Staff: Editor-in-Chief: at Vanderbilt’s Landing to Tottenville. An in- until 1948 was the Perth Amboy to South Bernard Linder spection trip from Vanderbilt’s Landing to Ferry route, which was rerouted from Perth Tri-State News and Eltingville was held for officials and stock- Amboy to Tottenville. -

International Camellia Journal 2010 No
AN OFFICIAL PUBLICATION OF 2010 I NTERNATIONAL CAMELLIA JOURNAL 2010 JOURNAL CAMELLIA NTERNATIONAL INTERNATIONAL CAMELLIA SOCIETY INTERNATIONAL NUMBER ISSN 0159-656X INTERNATIONAL CAMELLIA JOURNAL 国际山茶杂志 国際 ツノヾキ会誌 JOURNAL INTERNATIONAL DU CAMELLIA REVISTA INTERNAZIONALE DELLA CAMELIA REVISTA INTERNACIONAL DE LA CAMELIA INTERNATIONALE KAMELIENZEITSCHRIFT INTERNATIONAL CAMELLIA TIJDSCHRIFT Main Photo: Katsuhiko Mizuno. Inset: �hi���������eo Matsu�oto ‘Jikkô’(literally meaning ‘the sunlight’) is a 300 year old camellia just inside the entrance to the garden of Reikanji Temple in Kyoto City. It is thought to be the original plant of this variety and was cherished by the retired Emperor Gomizuno’o (1596-1680) and designated as a natural treasure by Kyoto City. See page 104 for Kentaro Nakamura’s paper that includes information about experiments for the propagation of this historic camellia. FRONT COVER PICTURE ‘Goshiki-yae-chiri-tsubaki’ was seen on several occasions on visits during the 2010 International Camellia Society Congress in Japan. The name means, literally “Five colours, double, petals scattering”. The five colours are all seen on one tree, with branches bearing white, deep pink, pale pink, striped pink on a white background, and striped with white on a pink background, making a glorious display. The most striking trees are ancient, estimated to be 400 – 500 years old. Its history is not clear, but there is a legend that the plant of the same cultivar at Jizoin Temple in camellia japonica camellia seeds filtered camellia oil Kyoto was brought in from Korea during the war between Japan and Korea in 1593. This unique cultivar the pride of the people of Kyoto and Nara. -

Itinerary Japan
Transportation By Train Date Destination From To Remark w/o JR Pass JR Pass Station Track Time Station Track Time TOTAL Monday, 16 Nov 15 Ueno Park Narita Int Airport 09.15 Tokyo 1 10.17 Ltd. Exp Narita Express 8 ¥ 2,820 ¥ ‐ Tokyo 7 10.26 Ueno 6 10.31 JR Joban Line for TSUCHIURA Sightseeing Ueno 12.36 Akihabara 12.39¥ 140 ¥ ‐ JR Yamanote Line for OSAKI Osaka Akihabara 14.20 Tokyo 14.23 JR Keihin‐Tohoku/Negishi Line Rapid for ISOGO Tokyo 17 14.33 Shin‐Osaka 24 17.26 Shinkansen Hikari 517 Shin‐Osaka 16 17.38 Osaka 4 17.42 ¥ 13,940 ¥ ‐ JR Special Rapid Service for BANSHUAKO Osaka 2 17.45 Kyobashi (Osaka) 17.51 JR Kansai Airport Rapid Service for KYOBASHI Kyobashi (Osaka) 17.53 Teradacho 18.04 Osaka Loop Line for TENNOJI Shinsaibashi Shopping Street Teradacho 19.01 Tennoji 14 19.03 ¥ 120 ¥ ‐ Osaka Loop Line for TENNOJI Tennoji 19.09 Shinsaibashi 19.17 ¥ 240 ¥ 240 Osaka City Subway Midosuji Line for SHIN‐OSAKA Rest at Hotel Shinsaibashi 21.05 Tennoji 21.14 ¥ 240 ¥ 240 Osaka City Subway Midosuji Line for TENNOJI Tennoji 11 21.20 Teradacho 21.22 ¥ 120 ¥ ‐ Osaka Loop Line for OSAKA TOTAL ¥ 17,620 ¥ 480 Tuesday, 17 Nov 15 Osaka Castle Teradacho 08.33 Osakajokoen 08.44 ¥ 160 ¥ ‐ Osaka Loop Line for SAKURAJIMA Kiyomizudera Osakajokoen 12.03 Osaka 1 12.12 ¥ 160 ¥ ‐ Osaka Loop Line for OSAKA Drop Luggage at Locker Coin of Osaka Station Osaka 7 12.26 Shin‐Osaka 14 12.30 JR Kyoto Line Local for TAKATSUKI ¥ 2,610 ¥ ‐ Shin‐Osaka 25 12.40 Kyoto 11 12.55 Shinkansen Hikari 468 Kyoto 9 13.07 Tofukuji 13.09 ¥ 140 ¥ ‐ JR Nara Line for JOYO Tofukuji 13.13 Kiyomizu‐gojo 13.16 ¥ 150 ¥ 150 Keihan Main Line Sub‐Exp. -

Junmai Ginjo MOMOKAWA 90 Ruby PTS Junmai Ginjo Craft Can Mildly Sweet with Hints of Red Berries, Cherry, a Medium-Dry, Crisp Saké with Fresh Aromas of Guava and Mochi
CRAFT BREWED IN THE PACIFIC NW AMERICA’S ORIGINAL CRAFT BREWERY VEGAN-FRIENDLY | GLUTEN-FREE | KOSHER NEW MOMOKAWA PRODUCT - Junmai Ginjo MOMOKAWA 90 Ruby PTS Junmai Ginjo Craft Can Mildly sweet with hints of red berries, cherry, A medium-dry, crisp saké with fresh aromas of guava and mochi. Hints of tropical fruit, melon and grapes on the nose. 92 melon, green apple and anise and subtle hints PTS of citrus and honeydew. Polish 58% ABV 14.8% SMV -3 Polish 58% ABV 14.1% SMV +4 Rice Exclusive California Calrose Rice Exclusive California Calrose Yeast Proprietary Enjoy chilled Yeast Proprietary Enjoy chilled AWARD 90pts, Exceptional, Best Buy, Gold Medal AWARD 92pts, Great Value, Gold Medal - Ultimate Wine Challenge ‘19 - World Saké Challenge ‘17 UPC: 250ml 7 47846 12250 2 UPC: 750ML 7 47846 13720 9 MOMOKAWA MOMOKAWA Organic - Junmai Ginjo SILVER Silver - Junmai Ginjo Notes of refreshing melon and lime combine SILVER Light, crisp and dry mouthfeel with hints of SILVER with delicate pineapple and cola flavors. mineral and citrus. Green apple, melon and spice on the nose. Polish 58% ABV 14.5% SMV -2 Rice Exclusive California Calrose Polish 58% ABV 14.8% SMV +7 Yeast Proprietary Enjoy chilled Rice Exclusive California Calrose Yeast Proprietary Enjoy chilled USDA CERTIFIED ORGANIC AWARDS Silver Medal - London Saké Challenge ‘19; AWARD Silver Medal - Rodeo Uncorked Int’l Wine Competition ‘19 Silver Medal - Tokyo Saké Competition ‘19 UPC: 300ML 7 47846 40051 8, 750ML 7 47846 40050 1, UPC: 750ML 7 47846 12720 0 19.5 L Keg 7 47846 40052 5 MOMOKAWA MOMOKAWA 94 Diamond - Junmai Ginjo 94 PTS PTS Organic - Junmai Ginjo Nigori 92 Medium-dry and crisp with a balance of soft Rich and silky layers of coconut and cream with a PTS water notes and fall flavors of apple and pear. -

Aachi Wa Ssipak Afro Samurai Afro Samurai Resurrection Air Air Gear
1001 Nights Burn Up! Excess Dragon Ball Z Movies 3 Busou Renkin Druaga no Tou: the Aegis of Uruk Byousoku 5 Centimeter Druaga no Tou: the Sword of Uruk AA! Megami-sama (2005) Durarara!! Aachi wa Ssipak Dwaejiui Wang Afro Samurai C Afro Samurai Resurrection Canaan Air Card Captor Sakura Edens Bowy Air Gear Casshern Sins El Cazador de la Bruja Akira Chaos;Head Elfen Lied Angel Beats! Chihayafuru Erementar Gerad Animatrix, The Chii's Sweet Home Evangelion Ano Natsu de Matteru Chii's Sweet Home: Atarashii Evangelion Shin Gekijouban: Ha Ao no Exorcist O'uchi Evangelion Shin Gekijouban: Jo Appleseed +(2004) Chobits Appleseed Saga Ex Machina Choujuushin Gravion Argento Soma Choujuushin Gravion Zwei Fate/Stay Night Aria the Animation Chrno Crusade Fate/Stay Night: Unlimited Blade Asobi ni Iku yo! +Ova Chuunibyou demo Koi ga Shitai! Works Ayakashi: Samurai Horror Tales Clannad Figure 17: Tsubasa & Hikaru Azumanga Daioh Clannad After Story Final Fantasy Claymore Final Fantasy Unlimited Code Geass Hangyaku no Lelouch Final Fantasy VII: Advent Children B Gata H Kei Code Geass Hangyaku no Lelouch Final Fantasy: The Spirits Within Baccano! R2 Freedom Baka to Test to Shoukanjuu Colorful Fruits Basket Bakemonogatari Cossette no Shouzou Full Metal Panic! Bakuman. Cowboy Bebop Full Metal Panic? Fumoffu + TSR Bakumatsu Kikansetsu Coyote Ragtime Show Furi Kuri Irohanihoheto Cyber City Oedo 808 Fushigi Yuugi Bakuretsu Tenshi +Ova Bamboo Blade Bartender D.Gray-man Gad Guard Basilisk: Kouga Ninpou Chou D.N. Angel Gakuen Mokushiroku: High School Beck Dance in -
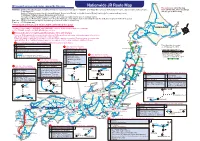
N JR Rote Map 4.1- 20160323
Wakkanai ◉Transport services and routes covered by this pass Nationwide JR Route Map The stations in red in the map Railways : JR lines throughout Japan, including Shinkansen “bullet trains” (except for “NOZOMI” and “MIZUHO” services), limited express trains, express trains and local trains. have JAPAN RAIL PASS exchange Tokyo Monorail offices. (as of March 2016) Aoimori Railway between Hachinohe and Aomori, Aomori and Noheji, or Hachinohe and Noheji (valid only for use on ordinary trains) IR Ishikawa Railway between Kanazawa and Tsubata Ainokaze Toyama Railway between Toyama and Takaoka (valid only for use on ordinary trains) (On Aoimori, IR Ishikawa, and Ainokaze Toyama Railways, valid only for travel extending through the indicated segments without stopover) Nayoro Buses : JR Bus services (except for expressway buses and some local services). Fukagawa Kamikawa Ferry : JR Miyajima ferry Mashike Asahikawa Abashiri Kitami ◉Transport services and routes NOT covered by this pass Biei Shiretoko-Shari Otaru Furano HOKKAIDO This pass is not valid for “NOZOMI” and “MIZUHO” services (both reserved and non-reserved) Sapporo Mashu on the Tōkaidō, Sanyō, and Kyūshū Shinkansen lines. Kutchan New-Chitose Oiwake Shibecha Nemuro Oshamambe Toya Airport Trains and services requiring additional basic fares and charges Minami-Chitose Travel on JR Group trains that connect directly with non-JR Group railways will require additional payment of basic fares Tomakomai Obihiro Kushiro and charges for the sections operated by the other company. Noboribetsu Please pay either on the train or at a station on the line of the company concerned. (The map below shows the main Muroran applicable train lines. Additional payment may also be required on non-regular services in addition to these.) Shin-Hakodate-Hokuto Goryōkaku Key to lists of trains and sections requiring additional basic fares and charges Kikonai Hakodate Samani This information is current MimmayaOminato as of February 26, 2016. -

Ichiju Sansai
Washoku was Registered by UNESCO as an Intangible Cultural Asset, But What is It? Contents The Four Characteristics of Washoku Culture Washoku was Registered by UNESCO as an Intangible Cultural Asset, But What is It? Diverse, fresh ingredients, The Four Characteristics of Washoku Culture and respect for their What is Washoku as Cuisine? individual flavors The land of Japan extends a Washoku Ingredients long way from north to south, • Rice, Vegetables, Seafood, Wagyu and is covered by an expressive expanse of nature through seas, Washoku Condiments mountains, and villages. Diverse ingredients with local roots are Dashi used in each part of the country, Fermented Condiments and preparation techniques and implements have developed to • Soy Sauce, Miso, Sake, Vinegar, Mirin, make the most of their flavors. Fish sauce Yakumi • Wasabi (Japanese horseradish), Shoga (ginger), Negi (green onions), Shiso (perilla), Yuzu (Japanese citron) Nutritional balance Washoku Style to support a healthy Ichiju sansai diet The diet based on ichiju sansai Cha-kaiseki and Kaiseki (one soup and three dishes) makes it easy to get a good Nihonshu (Japanese sake) nutritional balance, makes the most of the umami of dashi stock Wagashi (Japanese cakes) and Nihoncha and of fermented ingredients, (Japanese tea) and keeps down the intake of animal fats. That helps the Chopsticks and the Manners of Eating With Japanese people live long and Them resist obesity. • Ohashi • Manners of eating Expression of the beauty of nature and the changing seasons Dishes are decorated with items Definition such as seasonal flowers and leaves, and furnishings and “Washoku”, as registered by UNESCO, goes utensil are used that match beyond the food itself, referring to Japan’s the season.