The Role of Microorganisms in the Nutrient Cycling of Subtropical Savannah Soils
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
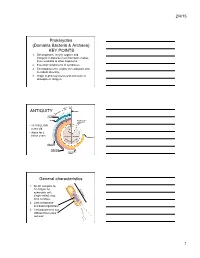
Prokaryotes (Domains Bacteria & Archaea)
2/4/15 Prokaryotes (Domains Bacteria & Archaea) KEY POINTS 1. Decomposers: recycle organic and inorganic molecules in environment; makes them available to other organisms. 2. Essential components of symbioses. 3. Encompasses the origins of metabolism and metabolic diversity. 4. Origin of photosynthesis and formation of atmospheric Oxygen Ceno- Meso- zoic zoic ANTIQUITY Humans Paleozoic Colonization of land Animals Origin of solar system and Earth • >3.5 BILLION years old. • Alone for 2 1 4 billion years Proterozoic Archaean Prokaryotes Billions of 2 years ago3 Multicellular eukaryotes Single-celled eukaryotes Atmospheric oxygen General characteristics 1. Small: compare to 10-100µm for 0.5-5µm eukaryotic cell; single-celled; may form colonies. 2. Lack membrane- enclosed organelles. 3. Cell wall present, but different from plant cell wall. 1 2/4/15 General characteristics 4. Occur everywhere, most numerous organisms. – More individuals in a handful of soil then there are people that have ever lived. – By far more individuals in our gut than eukaryotic cells that are actually us. General characteristics 5. Metabolic diversity established nutritional modes of eukaryotes. General characteristics 6. Important decomposers and recyclers 2 2/4/15 General characteristics 6. Important decomposers and recyclers • Form the basis of global nutrient cycles. General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • About 1/2 of all human diseases are caused by Bacteria General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • Extremely important in agriculture as well. Pierce’s disease is caused by Xylella fastidiosa, a Gamma Proteobacteria. It causes over $56 million in damage annually in California. That’s with $34 million spent to control it! = $90 million in California alone. -

Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas Sp
microorganisms Article Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas sp. AAP5 Karel Kopejtka 1 , Yonghui Zeng 1,2, David Kaftan 1,3 , Vadim Selyanin 1, Zdenko Gardian 3,4 , Jürgen Tomasch 5,† , Ruben Sommaruga 6 and Michal Koblížek 1,* 1 Centre Algatech, Institute of Microbiology, Czech Academy of Sciences, 379 81 Tˇreboˇn,Czech Republic; [email protected] (K.K.); [email protected] (Y.Z.); [email protected] (D.K.); [email protected] (V.S.) 2 Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, 1871 Frederiksberg C, Denmark 3 Faculty of Science, University of South Bohemia, 370 05 Ceskˇ é Budˇejovice,Czech Republic; [email protected] 4 Institute of Parasitology, Biology Centre, Czech Academy of Sciences, 370 05 Ceskˇ é Budˇejovice,Czech Republic 5 Research Group Microbial Communication, Technical University of Braunschweig, 38106 Braunschweig, Germany; [email protected] 6 Laboratory of Aquatic Photobiology and Plankton Ecology, Department of Ecology, University of Innsbruck, 6020 Innsbruck, Austria; [email protected] * Correspondence: [email protected] † Present Address: Department of Molecular Bacteriology, Helmholtz-Centre for Infection Research, 38106 Braunschweig, Germany. Abstract: An aerobic, yellow-pigmented, bacteriochlorophyll a-producing strain, designated AAP5 Citation: Kopejtka, K.; Zeng, Y.; (=DSM 111157=CCUG 74776), was isolated from the alpine lake Gossenköllesee located in the Ty- Kaftan, D.; Selyanin, V.; Gardian, Z.; rolean Alps, Austria. Here, we report its description and polyphasic characterization. Phylogenetic Tomasch, J.; Sommaruga, R.; Koblížek, analysis of the 16S rRNA gene showed that strain AAP5 belongs to the bacterial genus Sphingomonas M. Characterization of the Aerobic and has the highest pairwise 16S rRNA gene sequence similarity with Sphingomonas glacialis (98.3%), Anoxygenic Phototrophic Bacterium Sphingomonas psychrolutea (96.8%), and Sphingomonas melonis (96.5%). -

A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and Their Association with the Endangered, Endemic Snail Physella Johnsoni
A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and their Association with the Endangered, Endemic Snail Physella johnsoni By Michael Bilyj A thesis submitted to the Faculty of Graduate Studies in partial fulfillment of the requirements for the degree of Master of Science Department of Microbiology Faculty of Science University of Manitoba Winnipeg, Manitoba October 2011 © Copyright 2011, Michael A. Bilyj 1 Abstract The seasonal population fluctuation of anoxygenic phototrophs and the diversity of cyanobacteria at the Sulphur Mountain thermal springs of Banff, Canada were investigated and compared to the drastic population changes of the endangered snail Physella johnsoni. A new species and two strains of Rhodomicrobium were taxonomically characterized in addition to new species of Rhodobacter and Erythromicrobium. Major mat-forming organisms included Thiothrix-like species, oxygenic phototrophs of genera Spirulina, Oscillatoria, and Phormidium and purple nonsulfur bacteria Rhodobacter, Rhodopseudomonas and Rhodomicrobium. Aerobic anoxygenic phototrophs comprised upwards of 9.6 x 104 CFU/cm2 of mat or 18.9% of total aerobic heterotrophic bacterial isolates at certain sites, while maximal purple nonsulfur and purple sulfur bacteria were quantified at 3.2 x 105 and 2.0 x 106 CFU/cm2 of mat, respectively. Photosynthetic activity measurements revealed incredibly productive carbon fixation rates averaging 40.5 mg C/cm2/24 h. A temporal mismatch was observed for mat area and prokaryote-based organics to P. johnsoni population flux in a ―tracking inertia‖ manner. 2 Acknowledgements It is difficult to express sufficient gratitude to my supervisor Dr. Vladimir Yurkov for his unfaltering patience, generosity and motivation throughout this entire degree. -

Ameliorating Process Parameters for Zeaxanthin Yield in Arthrobacter Gandavensis MTCC 25325
Ram et al. AMB Expr (2020) 10:69 https://doi.org/10.1186/s13568-020-01008-4 ORIGINAL ARTICLE Open Access Ameliorating process parameters for zeaxanthin yield in Arthrobacter gandavensis MTCC 25325 Shristi Ram1,2, Sushma Rani Tirkey1,2, Madhava Anil Kumar1,3 and Sandhya Mishra1,2* Abstract The present study aims to escalate the production of prophylactic agent zeaxanthin using a screened potential bacterial isolate. For this purpose, a freshwater bacterium capable of producing zeaxanthin was isolated from Bor Talav, Bhavnagar. The 16S rRNA sequence confrmed the isolate as Arthrobacter gandavensis. The bacterium was also submitted to Microbial Type Culture Collection, CSIR-Institute of Microbial Technology, Chandigarh, India, with the accession number MTCC 25325. The chemo-metric tools were employed to optimise the infuencing factors such as pH, temperature, inoculum size, agitation speed, carbon source and harvest time on zeaxanthin yield. Thereafter, six parameters were narrowed down to three factors and were optimised using the central composite design (CCD) matrix. Maximum zeaxanthin (1.51 mg/g) was derived when A. gandavensis MTCC 25325 was grown under pH 6.0, 1.5% (w/v) glucose and 10% (v/v) inoculum size. A high regression coefcient (R2 0.92) of the developed model indicated the accurateness of the tested parameters. To the best of our knowledge,= this is the frst report on tailoring the process parameters using chemo-metric optimisation for escalating the zeaxanthin production by A. gandavensis MTCC 25325. Keywords: Arthrobacter gandavensis MTCC 25325, Central composite design, Nutraceutical, Zeaxanthin Key points Introduction Te pursuit of alternative sources as functional food (that • Chemo-metric approach was used for optimising serves the dual purpose of nutrition and diet) has led to zeaxanthin production. -

Pigments Produced by the Bacteria Belonging to the Genus Arthrobacter Nuthathai Sutthiwong, Yanis Caro, Mireille Fouillaud, Philippe Laurent, A
Pigments produced by the bacteria belonging to the genus Arthrobacter Nuthathai Sutthiwong, Yanis Caro, Mireille Fouillaud, Philippe Laurent, A. Valla, Laurent Dufossé To cite this version: Nuthathai Sutthiwong, Yanis Caro, Mireille Fouillaud, Philippe Laurent, A. Valla, et al.. Pigments produced by the bacteria belonging to the genus Arthrobacter. 7th International Congress of Pigments in Food – New technologies towards health, through colors, Jun 2013, Novara, Italy. 2016. hal- 01397507 HAL Id: hal-01397507 https://hal.archives-ouvertes.fr/hal-01397507 Submitted on 16 Nov 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Public Domain Pigments produced by the bacteria belonging to the genus Arthrobacter Sutthiwong N.1, 2, Caro Y.2, Fouillaud M.2, Laurent P.3, Valla A.4, Dufossé L.2,5 1 Agricultural Technology Department, Thailand Institute of Scientific and Technological Research, Pathum Thani, Thailand 2 Laboratoire de Chimie des Substances Naturelles et des Sciences des Aliments, Université de La Réunion, ESIROI Agroalimentaire,Sainte-Clotilde, -

Aerobic Respiration
Life is based on redox • All energy generation in biological systems is due to redox (reduction-oxidation) reactions Aerobic Respiration: + - C6H12O6 + 6 H2O ==> 6 CO2 + 24 H +24 e oxidation electron donor (aka energy source) + - (O2+ 4H + 4e ==> 2H2O) x6 reduction electron acceptor --------------------------------------- C6H12O6 + 6 O2 ==> 6 CO2 + 6 H2O overall reaction (24 electrons) Types of bacterial metabolisms • While eukaryotes only reduce O2 and oxidize organic compounds, prokaryotes can use a variety of electron donors and acceptors, organic and inorganic. - • Aerobic respiration: e acceptor is O2 - • Anaerobic respiration: e acceptor is not O2 • Fermentation: e- donor and acceptor are organic molecules • Chemolithotrophy: e- donor and acceptor are inorganic molecules • Phototrophy: e- donor is light and e- acceptor is either organic or inorganic all microorganisms energy source? chemical light chemotroph phototroph carbon source? carbon source? organic organic CO CO compound 2 compound 2 chemoheterotroph chemoautotroph photoheterotroph photoautotroph e- acceptor? Nitrifying and sulfur- use H O to reduce CO ? oxidizing bacteria 2 2 green non-sulfur and O Other than O 2 2 purple non-sulfur bacteria anoxygenic oxygenic photosynthesis: photosynthesis: green sulfur and most bacteria Organic Inorganic cyanobacteria compound compound purple sulfur bacteria fermentative organism anaerobic respiration: nitrate, sulfate, Fe(III) Aerobic or anaerobic respiration Chemolithotrophy Important molecules Redox Electron Carrier: for example the -

Nitrogen and Phosphorus Limitation of Oceanic Microbial Growth During Spring in the Gulf of Aqaba
Vol. 56: 227–239, 2009 AQUATIC MICROBIAL ECOLOGY Printed September 2009 doi: 10.3354/ame01357 Aquat Microb Ecol Published online August 27, 2009 Contribution to AME Special 2 ‘Progress and perspectives in aquatic primary productivity’ OPEN ACCESS Nitrogen and phosphorus limitation of oceanic microbial growth during spring in the Gulf of Aqaba David J. Suggett1,*, Noga Stambler2, Ondrej Prá$il3, Zbigniew Kolber4, Antonietta Quigg5, Evaristo Vázquez-Domínguez6, Tamar Zohary7, Tom Berman7, David Iluz8, Orly Levitan2, Tracy Lawson1, Efrat Meeder9,10, Boaz Lazar9,10, Edo Bar-Zeev2, Hana Medova3, Ilana Berman-Frank8 1Department of Biological Sciences, University of Essex, Colchester CO4 3SQ, UK 2Department of Geography and Environment, Bar-Ilan University, Ramat-Gan 52900, Israel 3Photosynthesis Laboratory, Institute of Microbiology ASCR, Opatovicky mlyn, 379 81 Trˇ ebonˇ, Czech Republic 4Monterey Bay Aquarium Research Institute, 7700 Sandholdt Road, Moss Landing, California 95039, USA 5Department of Marine Biology and Oceanography, Texas A&M University, Galveston, Texas 77551, USA 6CSIC, Institut de Ciències del Mar, Passeig Marítim de la Barceloneta 39-43, 08003 Barcelona, Spain 7Kinneret Limnological Laboratory, Israel Oceanographic and Limnological Research, PO Box 447, Migdal 14950, Israel 8The Mina & Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan 52900, Israel 9The Interuniversity Institute for Marine Sciences Coral Beach, PO Box 469, 88103 Eilat, Israel 10Institute of Earth Sciences, The Hebrew University Edmond J. Safra Campus, Jerusalem 91904, Israel ABSTRACT: Bioassay experiments were performed to identify how growth of key groups within the mi- crobial community was simultaneously limited by nutrient (nitrogen and phosphorus) availability during spring in the Gulf of Aqaba’s oceanic waters. -

Draft Genome Sequence of Arthrobacter Sp. Strain UCD-GKA (Phylum Actinobacteria)
PROKARYOTES crossm Downloaded from Draft Genome Sequence of Arthrobacter sp. Strain UCD-GKA (Phylum Actinobacteria) Gregory N. Kincheloe,a Jonathan A. Eisen,a,b David A. Coila http://genomea.asm.org/ University of California, Davis Genome Center, Davis, California, USAa; Department of Evolution and Ecology and Department of Medical Microbiology and Immunology, University of California Davis, Davis, California, USAb ABSTRACT Here we present the draft genome of Arthrobacter sp. strain UCD-GKA. The assembly contains 4,930,274 bp in 33 contigs. This strain was isolated from the Received 29 November 2016 Accepted 1 December 2016 Published 9 February 2017 handle of a weight bar in the UC Davis Activities and Recreation Center. Citation Kincheloe GN, Eisen JA, Coil DA. 2017. Draft genome sequence of Arthrobacter sp. strain UCD-GKA (phylum Actinobacteria). embers of the genus Arthrobacter are aerobic, Gram-positive bacteria (1) primarily Genome Announc 5:e01599-16. https:// doi.org/10.1128/genomeA.01599-16. Mknown for switching between the bacilli and cocci shapes depending on the on February 28, 2017 by UNIVERSITY OF CALIFORNIA-DAVIS environmental conditions (2). They are commonly found in soil. Copyright © 2017 Kincheloe et al. This is an open-access article distributed under the terms Arthrobacter sp. strain UCD-GKA was isolated from a squatting bar located in the of the Creative Commons Attribution 4.0 weight room of the UC Davis Activities and Recreation Center. This was part of an International license. undergraduate research project to provide microbial reference genomes of bacteria Address correspondence to Jonathan A. Eisen, isolated from the built environment (http://www.microbe.net). -

Fermentation and Anaerobic Decomposition in a Hot Spring
Fermentation and anaerobic decomposition in a hot spring microbial mat by Karen Leigh Anderson A thesis submitted in partial fulfillment of requirements for the degree of Master of Science in Microbiology Montana State University © Copyright by Karen Leigh Anderson (1984) Abstract: Fermentation was investigated in a low sulfate hot spring microbial mat (Octopus Spring) according to current models on anaerobic decomposition. The mat was studied to determine what fermentation products accumulated, where in the mat they accumulated, and what factors affected their accumulation. Mat samples were incubated under dark anaerobic conditions to measure accumulation of fermentation products. Acetate and propionate (ca. 3:1) were the major products to accumulate in a 55°,C mat. Other products accumulated to a much lesser extent. Incubation of mat samples of varying thickness showed that fermentation occurred in the top 4mm of the mat. This has interesting implications for fermentative organisms in the mat due to the diurnal changes in mat oxygen concentrations. Fermentation measured in mat samples collected at various temperatures (50°,-70°C) showed acetate and propionate to be the major accumulation products. According to the interspecies hydrogen transfer model, the hydrogen concentration in a system affects the types of fermentation products produced. At a 65° C site, with natural high hydrogen levels, and at a 55°C site, with active methanogenesis, fermentation product accumulation was compared. There was a greater ratio of reduced fermentation products to acetate, with the exception of propionate, at 65°C. Ethanol accumulated at the 65°C site, as did lactate, though to a lesser extent. -

Biochemical and Phylogenetic Analyses of Psychrophilic Isolates Belonging to the Arthrobacter Subgroup and Description of Arthrobacter Psychrolactophilus, Sp
Arch Microbiol (1999) 171:355–363 © Springer-Verlag 1999 ORIGINAL PAPER Jennifer Loveland-Curtze · Peter P. Sheridan · Kevin R. Gutshall · Jean E. Brenchley Biochemical and phylogenetic analyses of psychrophilic isolates belonging to the Arthrobacter subgroup and description of Arthrobacter psychrolactophilus, sp. nov. Received: 17 December 1998 / Accepted: 12 March 1999 Abstract During our work on psychrophilic microorgan- their cold-active enzymes, particularly β-galactosidases. isms we obtained a large collection of new isolates. In or- During this work we have isolated numerous organisms der to identify six of these, we examined their growth pro- from geographically distant habitats ranging from Penn- perties, cell wall compositions, and their 16S rRNA gene sylvania farmlands to Antarctica. Many of these psy- sequences. The results showed that all of the isolates are chrophiles are gram-positive, non-spore-forming rods. gram-positive, aerobic, contain lysine in their cell walls, Four isolates (B7, D2, D5, and D10) were physiologically and belong to the high mol% G+C Arthrobacter subgroup. characterized and assigned to the genus Arthrobacter be- Phylogenetic analysis of the 16S rRNA genes grouped cause they are strict aerobes, have rod/coccus morpholog- five isolates obtained from a small geographical region ical cycles, and contain lysine in their cell walls (Love- into a monophyletic clade. Isolate B7 had a 16S rRNA se- land et al. 1994; DePrada et al. 1996). quence that was 94.3% similar to that of Arthrobacter Arthrobacter species are members of the high mol% polychromogenes and 94.4% similar to that of Arthro- G+C actinomycete-coryneform bacteria (Stackebrandt bacter oxydans. -

Bacterial Community Structure in a Sympagic Habitat Expanding with Global Warming: Brackish Ice Brine at 85€“90 °N
The ISME Journal (2019) 13:316–333 https://doi.org/10.1038/s41396-018-0268-9 ARTICLE Bacterial community structure in a sympagic habitat expanding with global warming: brackish ice brine at 85–90 °N 1,11 1,2 3 4 5,8 Beatriz Fernández-Gómez ● Beatriz Díez ● Martin F. Polz ● José Ignacio Arroyo ● Fernando D. Alfaro ● 1 1 2,6 5 4,7 Germán Marchandon ● Cynthia Sanhueza ● Laura Farías ● Nicole Trefault ● Pablo A. Marquet ● 8,9 10 10 Marco A. Molina-Montenegro ● Peter Sylvander ● Pauline Snoeijs-Leijonmalm Received: 12 February 2018 / Revised: 11 June 2018 / Accepted: 24 July 2018 / Published online: 18 September 2018 © International Society for Microbial Ecology 2018 Abstract Larger volumes of sea ice have been thawing in the Central Arctic Ocean (CAO) during the last decades than during the past 800,000 years. Brackish brine (fed by meltwater inside the ice) is an expanding sympagic habitat in summer all over the CAO. We report for the first time the structure of bacterial communities in this brine. They are composed of psychrophilic extremophiles, many of them related to phylotypes known from Arctic and Antarctic regions. Community structure displayed strong habitat segregation between brackish ice brine (IB; salinity 2.4–9.6) and immediate sub-ice seawater (SW; – 1234567890();,: 1234567890();,: salinity 33.3 34.9), expressed at all taxonomic levels (class to genus), by dominant phylotypes as well as by the rare biosphere, and with specialists dominating IB and generalists SW. The dominant phylotypes in IB were related to Candidatus Aquiluna and Flavobacterium, those in SW to Balneatrix and ZD0405, and those shared between the habitats to Halomonas, Polaribacter and Shewanella. -

Benemérita Universidad Autónoma De Puebla
BENEMÉRITA UNIVERSIDAD AUTÓNOMA DE PUEBLA INSTITUTO DE CIENCIAS CENTRO DE INVESTIGACIONES EN CIENCIAS MICROBIOLÓGICAS POSGRADO EN MICROBIOLOGÍA DIVERSIDAD BACTERIANA METILOTRÓFICA ASOCIADA A LA CACTÁCEA Neobuxbaumia macrocephala, ENDÉMICA EN RIESGO DE LA RESERVA TEHUACÁN CUICATLÁN TESIS QUE PARA OBTENER EL GRADO DE: DOCTORA EN CIENCIAS (MICROBIOLOGÍA) PRESENTA: MC MARÍA DEL ROCÍO BUSTILLOS CRISTALES ASESOR DE TESIS: DC LUIS ERNESTO FUENTES RAMÍREZ PUEBLA, PUE. AGOSTO, 2017 Índice RESUMEN………………………………………………………............................1 ABSTRACT………………………………………………………………………..2 INTRODUCCIÓN………………………………………………………………….3 ANTECEDENTES………………………………………………...........................10 JUSTIFICACIÓN………………………………………………………………….11 OBJETIVOS……………………………………………………………………….11 General…………………………………………………………………………...11 Particulares……………………………………………………………………… 11 MATERIALES Y MÉTODOS…………………………………………………….12 1. Zona de muestreo…………………………………………………………..12 2. Aislamiento………………………………………………………………...13 3. Caracterización Fenotípica………………………………………………....13 3.1 Morfología microscópica ……………………………………………... 13 3.2 Morfología colonial…………………………………………………… 13 3.3 Características bioquímicas y fisiológicas de los aislados……………. 13 3.3.1. Pruebas Bioquímicas……………………………………….. 14 3.3.2 Crecimiento en diferentes fuentes de carbono……………… 14 3.3.3. Determinación de condiciones óptimas de crecimiento……. 14 3.3.3.1 Crecimiento a diferentes temperaturas……………. 14 3.3.3.2. Crecimiento bacteriano a diferentes pH………….. 14 3.3.3.3. Tolerancia a la presencia de cloruro de sodio……..15 en el medio 3.3.4 Comportamiento antimicrobiano…………………………….15 3.3.5 Crecimiento en metanol dependiente de Ca2+ y Ce3+ 15 4. Caracterización genotípica 15 4.1 Extracción de DNA genómico 15 4.2 PCR para la amplificación del gen que codifica la subunidad 16S rRNA 16 4.3 Amplificación del gen que codifica la subunidad 23S rRNA 16 i 4.4 Determinación de genes que codifican para la enzima metanol deshidrogenasa 17 4.4.1 PCR para amplificar genes involucrados en la metilotrofía 17 4.4.2.