Convergent Recruitment of 5´-Hydroxylase Activities by CYP75B Flavonoid B-Ring 10 Hydroxylases for Tricin Biosynthesis in Medicago Legumes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New and Revised Plant Declarations in South Australia
Twentieth Australasian Weeds Conference New and revised plant declarations in South Australia David A. Cooke, Michaela A. Heinson and John G. Virtue Biosecurity SA, GPO Box 1671, Adelaide, South Australia 5001, Australia ([email protected]) Summary South Australia has conducted a com- • Notification – The presence and locations of the prehensive review of plant declarations under the declared plant must be reported to the regional Natural Resources Management Act 2004. Various NRM Authority by the owner of the land. sections of this Act allow for prohibition of sale and • Control – Land owners are required to take action road transport of declared plants, legal requirements to destroy or control certain declared plant species for landholder control and formal notification of their present on their property. NRM Authorities are presence. Declarations are based on risk assessment also responsible for controlling these declared and policies adopted for each species. The review plants on road reserves, and may have the power has occurred over four phases with 155 weed policies to recover costs of control from the adjoining revised or newly developed. Policies cover broad goals landowners. and objectives, state risk assessment and regional The first comprehensive review of plants declared management actions. under weeds legislation in South Australia in 20 years, There are currently 140 declared plants in SA with as described in Heinson et al. (2014), commenced in another five currently under consideration. These are 2010. The review was split into phases and is now in alisma (Alisma lanceolatum With.), coastal tea-tree its fourth and final phase. For each phase a revised (Leptospermum laevigatum (Gaertn.) F.Muell.), dune or new, state-level policy was prepared for a plant. -

Phytochemical-Constituents, Safety and Efficacy of Commonly Used Medicinal Plants for the Treatment of Malaria in Ethiopia-A Review
Pharmacy & Pharmacology International Journal Review Article Open Access Phytochemical-constituents, safety and efficacy of commonly used medicinal plants for the treatment of malaria in Ethiopia-a review Abstract Volume 7 Issue 6 - 2019 Background: Malaria is among the ten top leading causes of morbidity and mortality in children under-5 years. Due to the rise of drug-resistant parasites and limited therapeutic Tigist Abera, Rekik Ashebir, Hirut Basha, efficacy of the available drugs, there is a need to search novel antimalarial drugs from Eyob Debebe, Abiy Abebe, Asfaw Meresa, medicinal plants commonly utilized as traditional medicines. Traditional medicines Samuel Woldekidan are often more available, affordable, sometimes are perceived as more effective than Traditional and Modern Medicine Research Directorate, conventional antimalarial drugs, cultural acceptable and the relatively lower cost. Hence Ethiopian Public Health Institute, Ethiopia traditional medicine becomes the novel candidate for the search and development of drugs for the prevention and treatment of malaria. Correspondence: Tigist Abera, Traditional and Modern Medicine Research Directorate, Ethiopian Public Health Objective: The present study aimed to review phytochemical constitute, safety and efficacy Institute, Addis Ababa, Ethiopia, commonly used medicinal plants for malaria treatment in Ethiopia. Email Methods: A web-based literature search was done by using scientific databases including Received: October 07, 2019 | Published: November 26, 2019 Pub Med, Science -

Honey Bee Suite © Rusty Burlew 2015 Master Plant List by Scientific Name United States
Honey Bee Suite Master Plant List by Scientific Name United States © Rusty Burlew 2015 Scientific name Common Name Type of plant Zone Full Link for more information Abelia grandiflora Glossy abelia Shrub 6-9 http://plants.ces.ncsu.edu/plants/all/abelia-x-grandiflora/ Acacia Acacia Thorntree Tree 3-8 http://www.2020site.org/trees/acacia.html Acer circinatum Vine maple Tree 7-8 http://www.nwplants.com/business/catalog/ace_cir.html Acer macrophyllum Bigleaf maple Tree 5-9 http://treesandshrubs.about.com/od/commontrees/p/Big-Leaf-Maple-Acer-macrophyllum.htm Acer negundo L. Box elder Tree 2-10 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=a841 Acer rubrum Red maple Tree 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=275374&isprofile=1&basic=Acer%20rubrum Acer rubrum Swamp maple Tree 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=275374&isprofile=1&basic=Acer%20rubrum Acer saccharinum Silver maple Tree 3-9 http://en.wikipedia.org/wiki/Acer_saccharinum Acer spp. Maple Tree 3-8 http://en.wikipedia.org/wiki/Maple Achillea millefolium Yarrow Perennial 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=b282 Aesclepias tuberosa Butterfly weed Perennial 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=b490 Aesculus glabra Buckeye Tree 3-7 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=281045&isprofile=1&basic=buckeye -
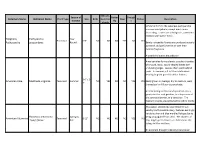
Common Name Botanical Name Alleghany
Attracts Season of Butter Drough Common Name Botanical Name Plant Type Size Birds Hummin Deer Native Description Interest fly t g birds Similar in form to the Japanese pachysandra one sees everywhere, except much more interesting. Leaves are a dull green, sometimes mottled with lighter flecks. Alleghany Pachysandra Year Perennial 6-8" NO NO NO YES NO YES Pachysandra procumbens Round Barely noticeable flowers are produced as early as March and perfume the air with their delicate fragrance. A wonderful native groundcover. American aloe forms a lovely succulent rosette of smooth, waxy, sword-shaped leaves with undulating edges. Leaves often sport reddish spots. In summer, a 3 to 5 foot stalk arises bearing fragrant greenish-white flowers. 3-6' x 2- American Aloe Manfreda virginica Perennial Summer NO YES NO NO YES YES Easily grown in average, dry to medium, well- 3' drained soil in full sun to part shade. An interesting architectural specimen, it is a good plant for rock gardens, in a dry corner of the perennial border, or a container. The fragrant blooms are pollinated by sphinx moths. This native, selected by Dale Hendrick's at nearby North Creek Nursery, features excitingly variable silver and blue marbled foliage due to Heuchera americana Spring to being propagated from seed. The clusters of American Alumroot Perennial 8-12" NO NO NO NO YES NO 'Dale's Strain' Fall tiny, bright green flowers are held above the foliage in May and June. An excellent drought tolerant groundcover. Viburnum trilobum is a native deciduous shrub to the northeastern and northwestern United States. -

Medical Ethnobotany of the Albanian Alps in Kosovo
Medical ethnobotany of the Albanian Alps in Kosovo Behxhet Mustafa, University of Prishtina Avni Hajdari, University of Prishtina Feriz Krasniqi, Kosovo Academy of Sciences and Arts Esat Hoxha, University of Prishtina Hatixhe Ademi, University of Prishtina Cassandra Quave, Emory University Andrea Pieroni, Univ Gastron Sci Journal Title: Journal of Ethnobiology and Ethnomedicine Volume: Volume 8 Publisher: BioMed Central | 2012-01-28, Pages 6-6 Type of Work: Article | Final Publisher PDF Publisher DOI: 10.1186/1746-4269-8-6 Permanent URL: https://pid.emory.edu/ark:/25593/rnh2w Final published version: http://dx.doi.org/10.1186/1746-4269-8-6 Copyright information: © 2012 Mustafa et al; licensee BioMed Central Ltd. Accessed September 26, 2021 3:06 AM EDT Mustafa et al. Journal of Ethnobiology and Ethnomedicine 2012, 8:6 http://www.ethnobiomed.com/content/8/1/6 JOURNAL OF ETHNOBIOLOGY AND ETHNOMEDICINE RESEARCH Open Access Medical ethnobotany of the Albanian Alps in Kosovo Behxhet Mustafa1, Avni Hajdari1*, Feriz Krasniqi2, Esat Hoxha1, Hatixhe Ademi1, Cassandra L Quave3 and Andrea Pieroni4 Abstract Background: Ethnobotanical studies are crucial in South-Eastern Europe for fostering local development and also for investigating the dynamics of Traditional Environmental Knowledge (TEK) related to plants in one of the most crucial European hotspots for biocultural diversity. The current medico-ethnobotanical survey was conducted in rural alpine communities in Kosovo. The aims of the study were twofold: 1) to document the state of TEK of medicinal plants in these communities; 2) to compare these findings with that of similar field studies previously conducted among local populations inhabiting the Montenegrin and Albanian side of the same Alpine range. -
Field Grown Cut Flowers
Nursery FACTSHEET September 2015 Field Grown Cut Flowers INTRODUCTION The culture of field grown flowers is an area of floriculture that is generating a lot of interest and is enjoying a steady growth rate. It provides a way to enter the floriculture industry without the $100 to $150 per square metre capital costs that are involved in some greenhouse crops. Recently, the largest area of growth has been in the specialty cut flowers as opposed to the more traditional field grown crops like statice, dahlias and gypsophila. As gardening increases in popularity, home consumers are becoming familiar with the many new and different flower species. In turn, consumers are starting to look for and demand these flowers in floral design work. Site Selection Whether you plan to lease or own the land, there are basic, yet important, site considerations (see Table 1). It is easier if you start with a suitable site rather than try to modify it later. Table 1. Considerations when selecting a production site Soil: It should be fertile and well drained. Soil tests are a basic management tool. Even if you are familiar with the soil in the area, it must be tested to determine pH, organic matter and nutrient levels. A pH of 6.0–6.5 is suitable for most cuts. Know the requirements of your crop before you make any major changes. Water: Good quality water must be available in sufficient quantities. Have the water source tested to determine essentials like pH and EC (salinity). Terrain: Flat land is easier to work. Watch out for low lying pockets that might be prone to early and late frosts, or flooding during the wet months. -

Live Bayside Plant Bayside Publication
Live Bayside Plant Bayside Contents 2 Introduction What are indigenous plants? Bayside’s original vegetation communities Bayside’s natural bushland reserves Get involved and learn 8 Bayside City Council Garden Design 76 Royal Avenue, Main considerations Sandringham. VIC 3191. Tel: 9599 4444 Habitat gardening Utilising runoff www.bayside.vic.gov.au Designing with indigenous plants 22 Acknowledgements Planting and Maintenance This booklet was produced by Green Gecko Publications with the kind permission of Plant selection Nillumbik Shire Council to modify Live Local Plant Local: A guide to planting in Nillumbik. Site preparation Photographs by Bayside City Council, Pauline Reynolds, Mary Trigger, Elaine Shallue, Planting technique Naina I Knoess Maintenance Design: www.nainak.com.au 28 Disclaimer: Although precautions have been taken to ensure the accuracy of the Indigenous Plant List information, the publishers, authors and printers cannot accept responsibility for any claim, Creepers and climbers loss, damage or liability arising out of the use of the information provided. Herbs and groundcovers Cover image: Love Creeper Grasses and flaxes This publication is printed on 100% recycled paperstock. Rushes and sedges Small shrubs Large shrubs Trees Pest Plants 61 Further Reading 65 Green Gecko PUBLICATIONS Mary Trigger Tel: 0414 641 337 Email: [email protected] ABN: 90618914198 Indigenous or native plants Many retail nurseries sell ‘native’ When two species crossbreed they plants. This refers to any plant found in can create a third species e.g. Horse x Introduction Australia, as opposed to an ‘indigenous’ Donkey = Mule. Many native Correas plant that is specific to a region e.g. have crossed with indigenous Correas Bayside. -

Bush Tucker Plant Fact Sheets
Traditional Bush Tucker Plant Fact Sheets Acknowledgements: We would like to acknowledge the traditional Noongar owners of this land and custodians of the knowledge used in these Fact Sheets. Illustrations and photos by Melinda Snowball, Deb Taborda, Amy Krupa, Pam Agar and Sian Mawson. ALGAE BUSTER Developed by SERCUL for use with the Bush Tucker Education Program. Used as food Used as medicine Used as resources Local to SW WA Caution: Do not prepare bush tucker food without having been shown by Indigenous or experienced persons. PHOSPHORUS www.sercul.org.au/our-projects/ AWARENESS PROJECT bushtucker/ Some bush tucker if eaten in large quantities or not prepared correctly can cause illness. Australian Bluebell Scientific name: Billardiera heterophylla Aboriginal name: Gumug (Noongar) Plant habit Leaf and stem Flower Fruit About ... Family PITTOSPORACEAE This plant relies on birds to eat the fruit and then Climate Temperate disperse the seeds. The seeds then germinate to produce a new plant. Habitat Open forest and woodland areas Australian bluebells are a common bushland plant Form Small shrub; twiner of the south west of Western Australia. This plant Height: up to 1.5 m has been introduced to the Eastern States, where it is considered a weed; as it forms a thick mat over the Foliage Long, leafy stems which twist around native vegetation. themselves or nearby plants Glossy green, leathery leaves The plant contains toxins which can cause nausea and Length: 50 mm skin irritation, so wear gloves if handling it. (Eurobodalla Shire Council) Flower Birak to Bunuru (Summer) but can flower all year around Intense blue Aboriginal Uses Bell-shaped Occur in clusters of two or more flowers • The fleshy blue berries can be eaten when ripe and Length: up to 10 mm are quite sweet with a soft texture Fruit Follow on from the flower Greenish-blue fruits Length: up to 20 mm Cylindrical in shape Contain many sticky seeds ALGAE BUSTER Developed by SERCUL for use with the Bush Tucker Education Program. -

Fragrant Annuals Fragrant Annuals
TheThe AmericanAmerican GARDENERGARDENER® TheThe MagazineMagazine ofof thethe AAmericanmerican HorticulturalHorticultural SocietySociety JanuaryJanuary // FebruaryFebruary 20112011 New Plants for 2011 Unusual Trees with Garden Potential The AHS’s River Farm: A Center of Horticulture Fragrant Annuals Legacies assume many forms hether making estate plans, considering W year-end giving, honoring a loved one or planting a tree, the legacies of tomorrow are created today. Please remember the American Horticultural Society when making your estate and charitable giving plans. Together we can leave a legacy of a greener, healthier, more beautiful America. For more information on including the AHS in your estate planning and charitable giving, or to make a gift to honor or remember a loved one, please contact Courtney Capstack at (703) 768-5700 ext. 127. Making America a Nation of Gardeners, a Land of Gardens contents Volume 90, Number 1 . January / February 2011 FEATURES DEPARTMENTS 5 NOTES FROM RIVER FARM 6 MEMBERS’ FORUM 8 NEWS FROM THE AHS 2011 Seed Exchange catalog online for AHS members, new AHS Travel Study Program destinations, AHS forms partnership with Northeast garden symposium, registration open for 10th annual America in Bloom Contest, 2011 EPCOT International Flower & Garden Festival, Colonial Williamsburg Garden Symposium, TGOA-MGCA garden photography competition opens. 40 GARDEN SOLUTIONS Plant expert Scott Aker offers a holistic approach to solving common problems. 42 HOMEGROWN HARVEST page 28 Easy-to-grow parsley. 44 GARDENER’S NOTEBOOK Enlightened ways to NEW PLANTS FOR 2011 BY JANE BERGER 12 control powdery mildew, Edible, compact, upright, and colorful are the themes of this beating bugs with plant year’s new plant introductions. -

Phylogeny and Phylogenetic Nomenclature of the Campanulidae Based on an Expanded Sample of Genes and Taxa
Systematic Botany (2010), 35(2): pp. 425–441 © Copyright 2010 by the American Society of Plant Taxonomists Phylogeny and Phylogenetic Nomenclature of the Campanulidae based on an Expanded Sample of Genes and Taxa David C. Tank 1,2,3 and Michael J. Donoghue 1 1 Peabody Museum of Natural History & Department of Ecology & Evolutionary Biology, Yale University, P. O. Box 208106, New Haven, Connecticut 06520 U. S. A. 2 Department of Forest Resources & Stillinger Herbarium, College of Natural Resources, University of Idaho, P. O. Box 441133, Moscow, Idaho 83844-1133 U. S. A. 3 Author for correspondence ( [email protected] ) Communicating Editor: Javier Francisco-Ortega Abstract— Previous attempts to resolve relationships among the primary lineages of Campanulidae (e.g. Apiales, Asterales, Dipsacales) have mostly been unconvincing, and the placement of a number of smaller groups (e.g. Bruniaceae, Columelliaceae, Escalloniaceae) remains uncertain. Here we build on a recent analysis of an incomplete data set that was assembled from the literature for a set of 50 campanulid taxa. To this data set we first added newly generated DNA sequence data for the same set of genes and taxa. Second, we sequenced three additional cpDNA coding regions (ca. 8,000 bp) for the same set of 50 campanulid taxa. Finally, we assembled the most comprehensive sample of cam- panulid diversity to date, including ca. 17,000 bp of cpDNA for 122 campanulid taxa and five outgroups. Simply filling in missing data in the 50-taxon data set (rendering it 94% complete) resulted in a topology that was similar to earlier studies, but with little additional resolution or confidence. -

Declared Plant
DECLARED PLANT Bluebell creeper Billardiera heterophylla January 2015 Bluebell creeper is a vine with twining stems, bearing blue, bell-shaped flowers. It takes on a shrubby habit in open situations. Grown as an ornamental plant in the temperate zones of Australia, it has become a garden escapee into native bushland. It is now declared under the Natural Resources Management Act 2004, with prohibition on sale throughout South Australia and enforced control in the Adelaide and Mount Lofty Ranges, Kangaroo Island and South East NRM regions. Other Common names: sollya, Western Australian bluebell creeper Family: Pittosporaceae Synonyms: Sollya heterophylla, Sollya heterophylla, Sollya erecta, Sollya fusiformis Origin: Native to south-western Western Australia WHY IS IT A PROBLEM? Bluebell creeper has spread beyond its native range as environmental weed. rapidly invades native and pine forests vigorous twining stems smother the native understorey and groundcover layer, also preventing regeneration of shrubs and trees regenerates readily from a persistent soil seed bank after disturbance or control DESCRIPTION Habit: vigorous climber reaching 3-4 m tall. Leaves: light to dark green, glossy and hairless varying from 2-5 cm long. They are alternately arranged, ranging from narrowly oblong to lance shaped. Stems: juvenile stems are reddish- brown, becoming woody as they mature. Flowers: pendant clusters of 2-5 small blue to mauve (sometimes pink or white), bell-shaped flowers 8-12 mm long. Flowering time mainly spring to summer. Fruit: succulent, green, cylindrical berries to 3.5 cm long, maturing to purplish green, containing numerous seeds 2-3 mm long. HOW IT SPREADS Seed is dispersed by birds and other animals such as foxes consuming fruit. -

False Flowers in Asteraceae
Available online at www.sciencedirect.com ScienceDirect Don’t be fooled: false flowers in Asteraceae Teng Zhang and Paula Elomaa The sunflower or daisy family, Asteraceae, comprises of meristems represent so-called flower unit meristems approximately 10% of all angiosperm species. Their (FUM) (Box 1) [4 ]. inflorescences form dense flower-like structures, pseudanthia or false flowers that may combine hundreds of individual In this review, we aim to highlight the most recent work flowers into a single structure. Recent data suggest that to understand the organization, patterning and develop- pseudanthia are analogs of single flowers not only ment of this unique structure and its role for adaptation. morphologically but also at developmental and genetic level, Because of still very limited number of molecular studies, and cannot merely be considered as condensed we also refer to selected older research addressing inflorescences. The large meristem size provides an advantage the major biological questions. Many of the floral traits to study basic principles of patterning as well as inflorescence in Asteraceae are of practical relevance for breeding of diversity in this evolutionary successful family. This knowledge commercially important crops within the family, includ- has also practical importance in the commercially important ing edible leaf, stem and seed oil crops (lettuce, artichoke, crops of the family. endive, sunflower, safflower), herbs and medicinal plants (Artemisia, Calendula, Echinaceae), as well as ornamental Address cut flowers (gerbera, chrysanthemum) [5]. Department of Agricultural Sciences, Viikki Plant Science Centre, 00014 University of Helsinki, Finland Origin and diversity of capitula in Asteraceae Corresponding author: Elomaa, Paula (paula.elomaa@helsinki.fi) Within flowering plants, Asteraceae belongs to the well- supported MGCA clade consisting of families of Menyanthaceae, Goodeniaceae, Calyceraceae, and Astera- Current Opinion in Plant Biology 2021, 59:101972 ceae (Figure 1).