Pharmacologic Activity of Phosphodiesterases and Their Inhibitors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biomolecules
CHAPTER 3 Biomolecules 3.1 Carbohydrates In the previous chapter you have learnt about the cell and 3.2 Fatty Acids and its organelles. Each organelle has distinct structure and Lipids therefore performs different function. For example, cell membrane is made up of lipids and proteins. Cell wall is 3.3 Amino Acids made up of carbohydrates. Chromosomes are made up of 3.4 Protein Structure protein and nucleic acid, i.e., DNA and ribosomes are made 3.5 Nucleic Acids up of protein and nucleic acids, i.e., RNA. These ingredients of cellular organelles are also called macromolecules or biomolecules. There are four major types of biomolecules— carbohydrates, proteins, lipids and nucleic acids. Apart from being structural entities of the cell, these biomolecules play important functions in cellular processes. In this chapter you will study the structure and functions of these biomolecules. 3.1 CARBOHYDRATES Carbohydrates are one of the most abundant classes of biomolecules in nature and found widely distributed in all life forms. Chemically, they are aldehyde and ketone derivatives of the polyhydric alcohols. Major role of carbohydrates in living organisms is to function as a primary source of energy. These molecules also serve as energy stores, 2021-22 Chapter 3 Carbohydrade Final 30.018.2018.indd 50 11/14/2019 10:11:16 AM 51 BIOMOLECULES metabolic intermediates, and one of the major components of bacterial and plant cell wall. Also, these are part of DNA and RNA, which you will study later in this chapter. The cell walls of bacteria and plants are made up of polymers of carbohydrates. -

Site-Selective Artificial Ribonucleases: Renaissance of Oligonucleotide Conjugates for Irreversible Cleavage of RNA Sequences
molecules Review Site-Selective Artificial Ribonucleases: Renaissance of Oligonucleotide Conjugates for Irreversible Cleavage of RNA Sequences Yaroslav Staroseletz 1,†, Svetlana Gaponova 1,†, Olga Patutina 1, Elena Bichenkova 2 , Bahareh Amirloo 2, Thomas Heyman 2, Daria Chiglintseva 1 and Marina Zenkova 1,* 1 Laboratory of Nucleic Acids Biochemistry, Institute of Chemical Biology and Fundamental Medicine SB RAS, Lavrentiev’s Ave. 8, 630090 Novosibirsk, Russia; [email protected] (Y.S.); [email protected] (S.G.); [email protected] (O.P.); [email protected] (D.C.) 2 School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Oxford Rd., Manchester M13 9PT, UK; [email protected] (E.B.); [email protected] (B.A.); [email protected] (T.H.) * Correspondence: [email protected]; Tel.: +7-383-363-51-60 † These authors contributed equally to this work. Abstract: RNA-targeting therapeutics require highly efficient sequence-specific devices capable of RNA irreversible degradation in vivo. The most developed methods of sequence-specific RNA cleav- age, such as siRNA or antisense oligonucleotides (ASO), are currently based on recruitment of either intracellular multi-protein complexes or enzymes, leaving alternative approaches (e.g., ribozymes Citation: Staroseletz, Y.; Gaponova, and DNAzymes) far behind. Recently, site-selective artificial ribonucleases combining the oligonu- S.; Patutina, O.; Bichenkova, E.; cleotide recognition motifs (or their structural -

Dissertation Characterization Of
DISSERTATION CHARACTERIZATION OF CYCLIC NUCLEOTIDE PHOSPHODIESTERASES IN THE TRANSCRIPTOME OF THE CRUSTACEAN MOLTING GLAND Submitted by Nada Mukhtar Rifai Department of Biology In partial fulfillment of the requirements For the Degree of Doctor of Philosophy Colorado State University Fort Collins, Colorado Spring 2019 Doctoral Committee: Advisor: Donald L. Mykles Deborah Garrity Shane Kanatous Santiago Di-Pietro Copyright by Nada Mukhtar Rifai 2019 All Rights Reserved ABSTRACT CHARACTERIZATION OF CYCLIC NUCLEOTIDE PHOSPHODIESTERASES IN THE TRANSCRIPTOME OF THE CRUSTACEAN MOLTING GLAND Molting in crustaceans is a complex physiological process that has to occur in order for the animal to grow. The old exoskeleton must be discarded and a new one to be formed from the inside out. Molting is coordinated and regulated mainly by two hormones; steroid hormones named ecdysteroids, which are synthesized and secreted from a pair of Y- organs (YOs) that are located in the cephalothorax and a neuropeptide hormone, the molt inhibiting hormone (MIH), which is secreted from the X-organ/sinus gland complex located in the eyestalks. Molting is induced when MIH is decreased in the blood (hemolymph) which in turn stimulates the YOs to produce and secrete ecdysteroids (molting hormones). There are four distinctive physiological states that the YO can be in throughout the molt cycle; the transition of the YO from the “basal” to the “activated” state happens when the animal enters premolt. During mid-premolt, the YO transitions to the “committed” state, in which the YO becomes insensitive to MIH. In this state, the circulating hemolymph contains high levels of ecdysteroids, which increase to a peak before the actual molt (ecdysis) happens. -

Phospholipase C-Related Catalytically Inactive Protein: a Novel Signaling Molecule for Modulating Fat Metabolism and Energy Expenditure
Journal of Oral Biosciences 61 (2019) 65e72 Contents lists available at ScienceDirect Journal of Oral Biosciences journal homepage: www.elsevier.com/locate/job Review Phospholipase C-related catalytically inactive protein: A novel signaling molecule for modulating fat metabolism and energy expenditure * Takashi Kanematsu a, b, , Kana Oue a, c, Toshiya Okumura a, Kae Harada a, 1, Yosuke Yamawaki a, 2, Satoshi Asano a, Akiko Mizokami d, Masahiro Irifune c, Masato Hirata e a Department of Cellular and Molecular Pharmacology, Division of Basic Life Sciences, Institute of Biomedical and Health Sciences, Hiroshima University, Hiroshima, 734-8553, Japan b Department of Cell Biology and Pharmacology, Faculty of Dental Science, Kyushu University, Fukuoka, 812-8582, Japan c Department of Dental Anesthesiology, Division of Applied Life Sciences, Institute of Biomedical and Health Sciences, Hiroshima University, Hiroshima, 734- 8553, Japan d OBT Research Center, Faculty of Dental Science, Kyushu University, Fukuoka, 812-8582, Japan e Fukuoka Dental College, Fukuoka, 814-0193, Japan article info abstract Article history: Background: Overweight and obesity are defined as excessive or abnormal fat accumulation in adipose Received 16 March 2019 tissues, and increase the risk of morbidity in many diseases, including hypertension, dyslipidemia, type 2 Received in revised form diabetes, coronary heart disease, and stroke, through pathophysiological mechanisms. There is strong 17 April 2019 evidence that weight loss reduces the risk of metabolic syndrome by limiting blood pressure and Accepted 19 April 2019 improving the levels of serum triglycerides, total cholesterol, low-density lipoprotein-cholesterol, and Available online 15 May 2019 high-density lipoprotein-cholesterol. To date, several attempts have been made to develop effective anti- obesity medication or weight-loss drugs; however, satisfactory drugs for clinical use have not yet been Keywords: Adipose tissue developed. -

Chemical Genetic Studies of Chemical Modulators of Mammalian Adenylyl Cyclases and Phosphodiesterases Expressed in Fission Yeast
Chemical Genetic Studies of Chemical Modulators of Mammalian Adenylyl Cyclases and Phosphodiesterases Expressed in Fission Yeast Author: Ana Santos de Medeiros Persistent link: http://hdl.handle.net/2345/bc-ir:106786 This work is posted on eScholarship@BC, Boston College University Libraries. Boston College Electronic Thesis or Dissertation, 2016 Copyright is held by the author, with all rights reserved, unless otherwise noted. Boston College Morrisey College of Arts and Sciences Graduate School Department of Biology CHEMICAL GENETIC STUDIES OF CHEMICAL MODULATORS OF MAMMALIAN ADENYLYL CYCLASES AND PHOSPHODIESTERASES EXPRESSED IN FISSION YEAST a dissertation by ANA SANTOS DE MEDEIROS Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy May 2016 © copyright by ANA SANTOS DE MEDEIROS 2016 ABSTRACT Cyclic adenosine monophosphate (cAMP) and the second messengers that modulate several biological processes are regulated by adenylyl cyclase (AC) and cyclic nucleotide phosphodiesterases (PDEs). ACs and PDEs are comprised of superfamilies of enzymes that are viewed as druggable targets due to their many distinct biological roles and tissue-specific distribution. As such, small molecule regulators of ACs and PDEs are important as chemical probes to study the roles of individual ACs or PDEs and as potential therapeutics. In the past, our lab has expressed 15 mammalian PDE genes in S. pombe, replacing the endogenous Cgs2 PDE. High throughput screens for PDE inhibitors identified novel compounds that show relevant biological activity in mammalian cell culture assays. The aim of this thesis is to develop tools to understand the mechanism of interaction between key regulators of the cAMP pathway and small molecules. -

Phosphodiesterase (PDE)
Phosphodiesterase (PDE) Phosphodiesterase (PDE) is any enzyme that breaks a phosphodiester bond. Usually, people speaking of phosphodiesterase are referring to cyclic nucleotide phosphodiesterases, which have great clinical significance and are described below. However, there are many other families of phosphodiesterases, including phospholipases C and D, autotaxin, sphingomyelin phosphodiesterase, DNases, RNases, and restriction endonucleases, as well as numerous less-well-characterized small-molecule phosphodiesterases. The cyclic nucleotide phosphodiesterases comprise a group of enzymes that degrade the phosphodiester bond in the second messenger molecules cAMP and cGMP. They regulate the localization, duration, and amplitude of cyclic nucleotide signaling within subcellular domains. PDEs are therefore important regulators ofsignal transduction mediated by these second messenger molecules. www.MedChemExpress.com 1 Phosphodiesterase (PDE) Inhibitors, Activators & Modulators (+)-Medioresinol Di-O-β-D-glucopyranoside (R)-(-)-Rolipram Cat. No.: HY-N8209 ((R)-Rolipram; (-)-Rolipram) Cat. No.: HY-16900A (+)-Medioresinol Di-O-β-D-glucopyranoside is a (R)-(-)-Rolipram is the R-enantiomer of Rolipram. lignan glucoside with strong inhibitory activity Rolipram is a selective inhibitor of of 3', 5'-cyclic monophosphate (cyclic AMP) phosphodiesterases PDE4 with IC50 of 3 nM, 130 nM phosphodiesterase. and 240 nM for PDE4A, PDE4B, and PDE4D, respectively. Purity: >98% Purity: 99.91% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 1 mg, 5 mg Size: 10 mM × 1 mL, 10 mg, 50 mg (R)-DNMDP (S)-(+)-Rolipram Cat. No.: HY-122751 ((+)-Rolipram; (S)-Rolipram) Cat. No.: HY-B0392 (R)-DNMDP is a potent and selective cancer cell (S)-(+)-Rolipram ((+)-Rolipram) is a cyclic cytotoxic agent. (R)-DNMDP, the R-form of DNMDP, AMP(cAMP)-specific phosphodiesterase (PDE) binds PDE3A directly. -
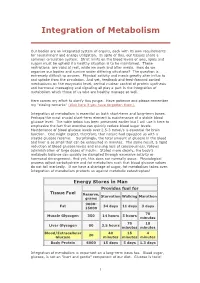
Integration of Metabolism
Integration of Metabolism Our bodies are an integrated system of organs, each with its own requirements for nourishment and energy utilization. In spite of this, our tissues share a common circulation system. Strict limits on the blood levels of ions, lipids and sugars must be upheld if a healthy situation is to be maintained. These restrictions are valid at rest, while we work and after meals. How do we organize our bodies and survive under differing situations? The question is extremely difficult to answer. Physical activity and meals greatly alter influx to and uptake from the circulation. And yet, feedback and feed-forward control mechanisms on the enzymatic level, central nuclear control of protein synthesis and hormonal messaging and signaling all play a part in the integration of metabolism which those of us who are healthy manage so well. Here comes my effort to clarify this jungle. Have patience and please remember my "closing remarks" (click here if you have forgotten them). Integration of metabolism is essential on both short-term and long-term bases. Perhaps the most crucial short-term element is maintenance of a stable blood glucose level. The table below has been presented earlier but I will use it here to emphasize the fact that exercise can quickly reduce blood sugar levels. Maintenance of blood glucose levels over 2.5-3 mmol/s is essential for brain function. One might expect, therefore, that nature had equipped us with a sizable glucose reserve. Surprisingly, the total amount of glucose in the blood and liver is so small that can be exhausted in minutes. -

Hypomethylation-Linked Activation of PLCE1 Impedes Autophagy and Promotes Tumorigenesis Through MDM2-Mediated Ubiquitination and Destabilization of P53
Author Manuscript Published OnlineFirst on February 17, 2020; DOI: 10.1158/0008-5472.CAN-19-1912 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Hypomethylation-linked activation of PLCE1 impedes autophagy and promotes tumorigenesis through MDM2-mediated ubiquitination and destabilization of p53 Yunzhao Chen1,3*, Huahua Xin1*, Hao Peng1*, Qi Shi1, Menglu Li1, Jie Yu3, Yanxia Tian1, Xueping Han1, Xi Chen1, Yi Zheng4, Jun Li5, Zhihao Yang1, Lan Yang1, Jianming Hu1, Xuan Huang2, Zheng Liu2, Xiaoxi Huang2, Hong Zhou6, Xiaobin Cui1**, Feng Li1,2** 1 Department of Pathology and Key Laboratory for Xinjiang Endemic and Ethnic Diseases, The First Affiliated Hospital, Shihezi University School of Medicine, Shihezi 832002, China; 2 Department of Pathology and Medical Research Center, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020, China; 3 The people's hospital of Suzhou National Hi-Tech District, Suzhou 215010, China; 4 Department of Gastroenterology, The First Affiliated Hospital, Shihezi University School of Medicine, Shihezi 832002, China; 5 Department of Ultrasound, The First Affiliated Hospital, Shihezi University School of Medicine, Shihezi 832002, China; 6 Bone Research Program, ANZAC Research Institute, The University of Sydney, Sydney, Australia. Running title: PLCE1 impedes autophagy via MDM2-p53 axis in ESCC *These authors contributed equally to this work. Corresponding authors: **Xiaobin Cui, Department of Pathology and Key Laboratory for Xinjiang Endemic and Ethnic Diseases, The First Affiliated Hospital, Shihezi University School of Medicine, Shihezi 832002, China. Phone: 86.0377.2850955; E-mail: [email protected]; **Feng Li, Department of Pathology and Medical Research Center, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020, China. -

Molecular Biology and Applied Genetics
MOLECULAR BIOLOGY AND APPLIED GENETICS FOR Medical Laboratory Technology Students Upgraded Lecture Note Series Mohammed Awole Adem Jimma University MOLECULAR BIOLOGY AND APPLIED GENETICS For Medical Laboratory Technician Students Lecture Note Series Mohammed Awole Adem Upgraded - 2006 In collaboration with The Carter Center (EPHTI) and The Federal Democratic Republic of Ethiopia Ministry of Education and Ministry of Health Jimma University PREFACE The problem faced today in the learning and teaching of Applied Genetics and Molecular Biology for laboratory technologists in universities, colleges andhealth institutions primarily from the unavailability of textbooks that focus on the needs of Ethiopian students. This lecture note has been prepared with the primary aim of alleviating the problems encountered in the teaching of Medical Applied Genetics and Molecular Biology course and in minimizing discrepancies prevailing among the different teaching and training health institutions. It can also be used in teaching any introductory course on medical Applied Genetics and Molecular Biology and as a reference material. This lecture note is specifically designed for medical laboratory technologists, and includes only those areas of molecular cell biology and Applied Genetics relevant to degree-level understanding of modern laboratory technology. Since genetics is prerequisite course to molecular biology, the lecture note starts with Genetics i followed by Molecular Biology. It provides students with molecular background to enable them to understand and critically analyze recent advances in laboratory sciences. Finally, it contains a glossary, which summarizes important terminologies used in the text. Each chapter begins by specific learning objectives and at the end of each chapter review questions are also included. -

Stimulation of Phospholipid Metabolism in Embryonic Muscle
Proc. Natl. Acad. Sci. USA Vol. 76, No. 9, pp. 4474-4478, September 1979 Cell Biology Stimulation of phospholipid metabolism in embryonic muscle cells treated with phospholipase C (phospholipid synthesis/myogenesis) CLAUDIA KENT Department of Biochemistry, Purdue University, West Lafayette, Indiana 47907 Communicated by Edwin T. Mertz, May 29, 1979 ABSTRACT Phospholipid metabolism is dramatically MATERIALS AND METHODS stimulated in cultured myogenic cells in which cell fusion was inhibited with phospholipase C (phosphatidylcholine choline- Cultured Cells. Pectoral muscle from 11-day chicken em- phosphohydrolase; EC 3.1.4.3). Phospholipase C was active bryos was dissected, loose connective tissue was removed, and under the culture conditions as shown by the degradation of the muscle was minced into 1- to 2-mm fragments. Cells were exogenous phosphatidylcholine. Rates of incorporation of 32p; dissociated from the tissue fragments by trituration with a and [metkyl-3Hlcholine into lipids were about 5-fold greater in phospholipase-treated cells than in either untreated fusing cells pasteur pipette (8) in calcium- and magnesium-free Earle's salt or untreated cells prevented from fusing by-calcium deprivation. solution. The cell suspension was filtered through cheesecloth, The greatest stimulation in the phospholipase C-treated cultures preplated for 15 min (9), and then diluted with culture medium occurred with synthesis of phospai tlcholine and sphin- to 5 X 105 cells/ml. The cells were plated in tissue culture dishes gomyelin; synthesis of phosphatidyinositol and cardiolipin was precoated with rat tail collagen (10) at 8 ml of cell suspension not stimulated. Degradation of cellular [32Plphosphatidylcholine and appearance in the culture medium of the degradation per 100 mm dish. -

Diagnostic Value of Serum Enzymes-A Review on Laboratory Investigations
Review Article ISSN 2250-0480 VOL 5/ ISSUE 4/OCT 2015 DIAGNOSTIC VALUE OF SERUM ENZYMES-A REVIEW ON LABORATORY INVESTIGATIONS. 1VIDYA SAGAR, M.SC., 2DR. VANDANA BERRY, MD AND DR.ROHIT J. CHAUDHARY, MD 1Vice Principal, Institute of Allied Health Sciences, Christian Medical College, Ludhiana 2Professor & Ex-Head of Microbiology Christian Medical College, Ludhiana 3Assistant Professor Department of Biochemistry Christian Medical College, Ludhiana ABSTRACT Enzymes are produced intracellularly, and released into the plasma and body fluids, where their activities can be measured by their abilities to accelerate the particular chemical reactions they catalyze. But different serum enzymes are raised when different tissues are damaged. So serum enzyme determination can be used both to detect cellular damage and to suggest its location in situ. Some of the biochemical markers such as alanine aminotransferase, aspartate aminotransferase, alkaline phasphatase, gamma glutamyl transferase, nucleotidase, ceruloplasmin, alpha fetoprotein, amylase, lipase, creatine phosphokinase and lactate dehydrogenase are mentioned to evaluate diseases of liver, pancreas, skeletal muscle, bone, etc. Such enzyme test may assist the physician in diagnosis and treatment. KEYWORDS: Liver Function tests, Serum Amylase, Lipase, CPK and LDH. INTRODUCTION mitochondrial AST is seen in extensive tissue necrosis during myocardial infarction and also in chronic Liver diseases like liver tissue degeneration DIAGNOSTIC SERUM ENZYME and necrosis². But lesser amounts are found in Enzymes are very helpful in the diagnosis of brain, pancreas and lung. Although GPT is plentiful cardiac, hepatic, pancreatic, muscular, skeltal and in the liver and occurs only in the small amount in malignant disorders. Serum for all enzyme tests the other tissues. -

GC-MS Metabolic Profile and -Glucosidase-, -Amylase-, Lipase-, and Acetylcholinesterase-Inhibitory Activities of Eight Peach
molecules Article GC-MS Metabolic Profile and α-Glucosidase-, α-Amylase-, Lipase-, and Acetylcholinesterase-Inhibitory Activities of Eight Peach Varieties Dasha Mihaylova 1,* , Ivelina Desseva 2,* , Aneta Popova 3 , Ivayla Dincheva 4 , Radka Vrancheva 2 , Anna Lante 5 and Albert Krastanov 1 1 Department of Biotechnology, Technological Faculty, University of Food Technologies, 4002 Plovdiv, Bulgaria; [email protected] 2 Department of Analytical Chemistry and Physical Chemistry, Technological Faculty, University of Food Technologies, 4002 Plovdiv, Bulgaria; [email protected] 3 Department of Catering and Tourism, Economics Faculty, University of Food Technologies, 4002 Plovdiv, Bulgaria; [email protected] 4 AgroBioInstitute, Agricultural Academy, 8 Dr. Tsankov Blvd., 1164 Sofia, Bulgaria; [email protected] 5 Department of Agronomy, Food, Natural Resources, Animals, and Environment—DAFNAE, Agripolis, University of Padova, 35020 Legnaro, Italy; [email protected] * Correspondence: [email protected] (D.M.); [email protected] (I.D.) Abstract: The inhibition of certain digestive enzymes by target food matrices represents a new approach in the treatment of socially significant diseases. Proving the ability of fruits to inhibit such enzymes can support the inclusion of specific varieties in the daily diets of patients with diabetes, obesity, Alzheimer’s disease, etc., providing them with much more than just valuable Citation: Mihaylova, D.; Desseva, I.; micro- and macromolecules. The current study aimed atidentifying and comparing the GC-MS Popova, A.; Dincheva, I.; Vrancheva, metabolic profiles of eight peach varieties (“Filina”, “Ufo 4, “Gergana”, “Laskava”, “July Lady”, R.; Lante, A.; Krastanov, A. GC-MS “Flat Queen”, “Evmolpiya”, and “Morsiani 90”) grown in Bulgaria (local and introduced) and to Metabolic Profile and α-Glucosidase-, evaluate the inhibitory potential of their extracts towards α-glucosidase, α-amylase, lipase, and α-Amylase-, Lipase-, and acetylcholinesterase.