To View Our Newest Offering on New Psychoactive Substances
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review of Market for Octane Enhancers
May 2000 • NREL/SR-580-28193 Review of Market for Octane Enhancers Final Report J.E. Sinor Consultants, Inc. Niwot, Colorado National Renewable Energy Laboratory 1617 Cole Boulevard Golden, Colorado 80401-3393 NREL is a U.S. Department of Energy Laboratory Operated by Midwest Research Institute • Battelle • Bechtel Contract No. DE-AC36-99-GO10337 May 2000 • NREL/SR-580-28193 Review of Market for Octane Enhancers Final Report J.E. Sinor Consultants, Inc. Niwot, Colorado NREL Technical Monitor: K. Ibsen Prepared under Subcontract No. TXE-0-29113-01 National Renewable Energy Laboratory 1617 Cole Boulevard Golden, Colorado 80401-3393 NREL is a U.S. Department of Energy Laboratory Operated by Midwest Research Institute • Battelle • Bechtel Contract No. DE-AC36-99-GO10337 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or any agency thereof. Available electronically at http://www.doe.gov/bridge Available for a processing fee to U.S. -

Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances 2017
INTERNATIONAL NARCOTICS CONTROL BOARD Precursors and chemicals frequently used in the illicit manufacture of narcotic drugs and psychotropic substances 2017 EMBARGO Observe release date: Not to be published or broadcast before Thursday, 1 March 2018, at 1100 hours (CET) UNITED NATIONS CAUTION Reports published by the International Narcotics Control Board in 2017 The Report of the International Narcotics Control Board for 2017 (E/INCB/2017/1) is supplemented by the following reports: Narcotic Drugs: Estimated World Requirements for 2018—Statistics for 2016 (E/INCB/2017/2) Psychotropic Substances: Statistics for 2016—Assessments of Annual Medical and Scientific Requirements for Substances in Schedules II, III and IV of the Convention on Psychotropic Substances of 1971 (E/INCB/2017/3) Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances: Report of the International Narcotics Control Board for 2017 on the Implementation of Article 12 of the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 (E/INCB/2017/4) The updated lists of substances under international control, comprising narcotic drugs, psychotropic substances and substances frequently used in the illicit manufacture of narcotic drugs and psychotropic substances, are contained in the latest editions of the annexes to the statistical forms (“Yellow List”, “Green List” and “Red List”), which are also issued by the Board. Contacting the International Narcotics Control Board The secretariat of the Board may be reached at the following address: Vienna International Centre Room E-1339 P.O. Box 500 1400 Vienna Austria In addition, the following may be used to contact the secretariat: Telephone: (+43-1) 26060 Fax: (+43-1) 26060-5867 or 26060-5868 Email: [email protected] The text of the present report is also available on the website of the Board (www.incb.org). -

Federal Register/Vol. 85, No. 167/Thursday, August 27, 2020
Federal Register / Vol. 85, No. 167 / Thursday, August 27, 2020 / Proposed Rules 52935 BIS does not seek to expand DATES: Comments must be submitted Drug Enforcement Administration, Attn: jurisdiction over technologies that are electronically or postmarked on or DEA Federal Register Representative/ not currently subject to the EAR, such before September 28, 2020. DPW, 8701 Morrissette Drive, as ‘‘fundamental research’’ described in Interested persons may file written Springfield, Virginia 22152. § 734.8 of the EAR. comments on this proposal in FOR FURTHER INFORMATION CONTACT: BIS will review public comments accordance with 21 CFR 1308.43(g). Scott A. Brinks, Regulatory Drafting and submitted in response to this ANPRM to Commenters should be aware that the Policy Support Section, Diversion help inform BIS and its interagency electronic Federal Docket Management Control Division, Drug Enforcement partners’ efforts to identify, reevaluate System will not accept comments after Administration; Mailing Address: 8701 and subsequently control foundational 11:59 p.m. Eastern Time on the last day Morrissette Drive, Springfield, Virginia technologies. This interagency process of the comment period. 22152; Telephone: (571) 362–8209. is expected to result in rules and Interested persons may file a request SUPPLEMENTARY INFORMATION: comment periods with new control for a hearing or waiver of hearing levels for items currently controlled for pursuant to 21 CFR 1308.44 and in Posting of Public Comments AT reasons on the CCL or new ECCNs accordance with 21 CFR 1316.45 and/or Please note that all comments on the CCL for technologies currently 1316.47, as applicable. Requests for a received in response to this docket are classified as EAR99. -

The 2006 Prohibited List International Standard
The World Anti-Doping Code THE 2006 PROHIBITED LIST INTERNATIONAL STANDARD The official text of the Prohibited List shall be maintained by WADA and shall be published in English and French. In the event of any conflict between the English and French versions, the English version shall prevail. This List shall come into effect on 1 January 2006. THE 2006 PROHIBITED LIST WORLD ANTI-DOPING CODE Valid 1 January 2006 The use of any drug should be limited to medically justified indications SUBSTANCES AND METHODS PROHIBITED AT ALL TIMES (IN- AND OUT-OF-COMPETITION) PROHIBITED SUBSTANCES S1. ANABOLIC AGENTS Anabolic agents are prohibited. 1. Anabolic Androgenic Steroids (AAS) a. Exogenous* AAS, including: 1-androstendiol (5α-androst-1-ene-3β,17β-diol ); 1-androstendione (5α- androst-1-ene-3,17-dione); bolandiol (19-norandrostenediol); bolasterone; boldenone; boldione (androsta-1,4-diene-3,17-dione); calusterone; clostebol; danazol (17α-ethynyl-17β-hydroxyandrost-4-eno[2,3-d]isoxazole); dehydrochlormethyltestosterone (4-chloro-17β-hydroxy-17α-methylandrosta- 1,4-dien-3-one); desoxymethyltestosterone (17α-methyl-5α-androst-2-en- 17β-ol); drostanolone; ethylestrenol (19-nor-17α-pregn-4-en-17-ol); fluoxymesterone; formebolone; furazabol (17β-hydroxy-17α-methyl-5α- androstano[2,3-c]-furazan); gestrinone; 4-hydroxytestosterone (4,17β-dihydroxyandrost-4-en-3-one); mestanolone; mesterolone; metenolone; methandienone (17β-hydroxy-17α- methylandrosta-1,4-dien-3-one); methandriol; methasterone (2α, 17α- dimethyl-5α-androstane-3-one-17β-ol); methyldienolone -

Organocatalytic Asymmetric N-Sulfonyl Amide C-N Bond Activation to Access Axially Chiral Biaryl Amino Acids
ARTICLE https://doi.org/10.1038/s41467-020-14799-8 OPEN Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids Guanjie Wang1, Qianqian Shi2, Wanyao Hu1, Tao Chen1, Yingying Guo1, Zhouli Hu1, Minghua Gong1, ✉ ✉ ✉ Jingcheng Guo1, Donghui Wei 2 , Zhenqian Fu 1,3 & Wei Huang1,3 1234567890():,; Amides are among the most fundamental functional groups and essential structural units, widely used in chemistry, biochemistry and material science. Amide synthesis and trans- formations is a topic of continuous interest in organic chemistry. However, direct catalytic asymmetric activation of amide C-N bonds still remains a long-standing challenge due to high stability of amide linkages. Herein, we describe an organocatalytic asymmetric amide C-N bonds cleavage of N-sulfonyl biaryl lactams under mild conditions, developing a general and practical method for atroposelective construction of axially chiral biaryl amino acids. A structurally diverse set of axially chiral biaryl amino acids are obtained in high yields with excellent enantioselectivities. Moreover, a variety of axially chiral unsymmetrical biaryl organocatalysts are efficiently constructed from the resulting axially chiral biaryl amino acids by our present strategy, and show competitive outcomes in asymmetric reactions. 1 Key Laboratory of Flexible Electronics & Institute of Advanced Materials, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, China. 2 College -
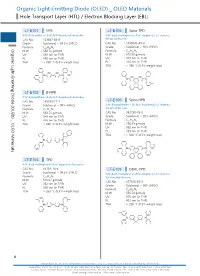
Organic Light-Emitting Diode (OLED) OLED Materials Hole Transport Layer (HTL) / Electron Blocking Layer (EBL)
Organic Light-EmittingOrganic Light-Emitting DiodeHole Transport(OLED) Layer (HTL) / Diode (OLED) _ OLED Materials Electron Blocking Layer (EBL) Organic Light-Emitting Diode (OLED) _ OLED Materials Hole Transport Layer (HTL) / Electron Blocking Layer (EBL) LT-E101 NPB LT-E105 Spiro-TPD N,N'-Bis(naphthalen-1-yl)-N,N'-bis(phenyl)-benzidine N,N'-Bis(3-methylphenyl)-N,N'-bis(phenyl)-2,7-diamino- CAS No. : 123847-85-8 9,9-spirobifluorene Grade : Sublimed, > 99.5% (HPLC) CAS No. : 1033035-83-4 : Grade : Sublimed, > 99% (HPLC) Organic Light-Emitting Diode (OLED) _ OLED Materials Formula C44H32N2 M.W. : 588.74 g/mole Formula : C51H38N2 UV : 339 nm (in THF) M.W. : 678.86 g/mole PL : 450 nm (in THF) UV : 379 nm (in THF) TGA : > 350 °C (0.5% weight loss) PL : 416 nm (in THF) TGA : > 280 °C (0.5% weight loss) N N N N LT-E102 β-NPB N,N'-Bis(naphthalen-2-yl)-N,N'-bis(phenyl)-benzidine Spiro-NPB CAS No. : 139255-17-7 LT-E106 Grade : Sublimed, > 99% (HPLC) N,N'-Bis(naphthalen-1-yl)-N,N'-bis(phenyl)-2,7-diamino- 9,9-spirobifluorene Formula : C44H32N2 M.W. : 588.74 g/mole CAS No. : 932739-76-9 UV : 349 nm (in THF) Grade : Sublimed, > 99% (HPLC) PL : 416 nm (in THF) Formula : C57H38N2 TGA : > 330 °C (0.5% weight loss) M.W. : 750.93 g/mole UV : 380 nm (in THF) PL : 453 nm (in THF) TGA : > 360 °C (0.5% weight loss) N N N N LT-E103 TPD N,N'-Bis(3-methylphenyl)-N,N'-bis(phenyl)-benzidine CAS No. -

Hydroxydopamine Lesions of the Nucleus Accumbens and the Mglur2/3 Agonist LY379268
Neuropsychopharmacology (2003) 28, 1440–1447 & 2003 Nature Publishing Group All rights reserved 0893-133X/03 $25.00 www.neuropsychopharmacology.org Toluene-Induced Locomotor Activity is Blocked by 6- Hydroxydopamine Lesions of the Nucleus Accumbens and the mGluR2/3 Agonist LY379268 1,3 2 ,1 AC Riegel , SF Ali , ED French* 1Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ, USA; 2Neurochemistry Laboratory, Division of Neurotoxicology, National Center for Toxicological Research/US FDA, Jefferson, AR, USA The abuse of volatile inhalants remains a prominent, yet poorly understood, form of substance abuse among youth. Nevertheless, the identification of a mechanism underlying the reinforcing properties of inhalants has been hampered by the lack of a clearly identifiable neural substrate upon which these chemicals act. One ingredient that is common to many abused inhalants is toluene, an organic solvent that is self-administered by nonhuman primates and rodents. Most drugs of abuse have been found to elicit forward locomotion in rats, an effect owing to the activation of mesoaccumbal dopamine (DA) pathways. Thus, the present study was undertaken using two different approaches to determine whether toluene-induced locomotor hyperactivity is also ultimately dependent upon DA neurotransmission in the mesolimbic nucleus accumbens (NAC). Here we report on the effects of 6-hydroxydopamine (6-OHDA) lesions of the NAC or pretreatment with the metabotropic mGlu2/3 receptor agonist LY379268 on toluene-induced locomotor activity. Both procedures, which are known to alter neurotransmission within the NAC, significantly attenuated toluene’s locomotor stimulatory effects. These results provide strong support for a central mechanism of action of inhalants, which in the past has been more typically attributed to general nonspecific mechanisms throughout the brain. -

Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-25-2018 Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected] DOI: 10.25148/etd.FIDC006565 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Chemistry Commons Recommended Citation Seither, Joshua Zolton, "Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances" (2018). FIU Electronic Theses and Dissertations. 3823. https://digitalcommons.fiu.edu/etd/3823 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida APPLICATION OF HIGH RESOLUTION MASS SPECTROMETRY FOR THE SCREENING AND CONFIRMATION OF NOVEL PSYCHOACTIVE SUBSTANCES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Joshua Zolton Seither 2018 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Joshua Zolton Seither, and entitled Application of High- Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Piero Gardinali _______________________________________ Bruce McCord _______________________________________ DeEtta Mills _______________________________________ Stanislaw Wnuk _______________________________________ Anthony DeCaprio, Major Professor Date of Defense: April 25, 2018 The dissertation of Joshua Zolton Seither is approved. -

Written Witness Statement for U.S. Sentencing Commission's Public
STATEMENT OF TERRENCE L. BOOS, PH.D. SECTION CHIEF DRUG AND CHEMICAL EVALUATION SECTION DIVERSION CONTROL DIVISION DRUG ENFORCEMENT ADMINISTRATION and CASSANDRA PRIOLEAU, PH.D. DRUG SCIENCE SPECIALIST DRUG AND CHEMICAL EVALUATION SECTION DIVERSION CONTROL DIVISION DRUG ENFORCEMENT ADMINISTRATION - - - BEFORE THE UNITED STATES SENTENCING COMMISSION - - - HEARING ON SENTENCING POLICY FOR SYNTHETIC DRUGS - - - OCTOBER 4, 2017 WASHINGTON, D.C. 1 Introduction New Psychoactive Substances (NPS) are substances trafficked as alternatives to well- studied controlled substances of abuse. NPS have demonstrated adverse health effects such as paranoia, psychosis, and seizures to name a few. Cathinones, cannabinoids, and fentanyl-related substances are the most common NPS drug classes encountered on the illicit drug market, all with negative consequences for the user to include serious injury and death. Our early experience saw substances being introduced from past research efforts in an attempt to evade controls. This has evolved to NPS manufacturers structurally altering substances at a rapid pace with unknown outcomes to targeting specific user populations. These substances represent an unprecedented level of diversity and consequences. Due to clandestine manufacture and unscrupulous trafficking, the user is at great risk. A misconception exists that these substances carry a lower risk of harm In reality, published reports from law enforcement, emergency room physicians and scientists, accompanied with autopsies from medical examiners, have clearly demonstrated the harmful and potentially deadly consequences of using synthetic cathinones. These substances are introduced in an attempt to circumvent drug controls and the recent flood of NPS remains a challenge for law enforcement and public health. The United Nations Office on Drugs and Crime reported over 700 NPS encountered.1 The manufacturers and traffickers make minor changes in the chemical structure of known substances of abuse and maintain the pharmacological effect. -

16.19.20 Nmac 1 Title 16 Occupational And
TITLE 16 OCCUPATIONAL AND PROFESSIONAL LICENSING CHAPTER 19 PHARMACISTS PART 20 CONTROLLED SUBSTANCES 16.19.20.1 ISSUING AGENCY: Regulation and Licensing Department - Board of Pharmacy. [16.19.20.1 NMAC - Rp 16.19.20.1 NMAC, 06-26-2018] 16.19.20.2 SCOPE: All persons or entities that manufacture, distribute, dispense, administer, prescribe, deliver, analyze, or conduct research using controlled substances. [16.19.20.2 NMAC - Rp 16.19.20.2 NMAC, 06-26-2018] 16.19.20.3 STATUTORY AUTHORITY: Section 30-31-11 of the Controlled Substances Act, 30-31-1 through 30-31-42 NMSA 1978, authorizes the board of pharmacy to promulgate regulations and charge reasonable fees for the registration and control of the manufacture, distribution and dispensing of controlled substances. [16.19.20.3 NMAC - Rp 16.19.20.3 NMAC, 06-26-2018] 16.19.20.4 DURATION: Permanent. [16.19.20.4 NMAC - Rp 16.19.20.4 NMAC, 06-26-2018] 16.19.20.5 EFFECTIVE DATE: June 26, 2018, unless a different date is cited at the end of a section. [16.19.20.5 NMAC - Rp 16.19.20.5 NMAC, 06-26-2018] 16.19.20.6 OBJECTIVE: The objective of Part 20 of Chapter 19 is to protect the public health and welfare of the citizens of New Mexico by controlling and monitoring access to controlled substances and to give notice of the board’s designation of particular substances as controlled substances. [16.19.20.6 NMAC - Rp 16.19.20.6 NMAC, 06-26-2018] 16.19.20.7 DEFINITIONS: [RESERVED] [16.19.20.7 NMAC - Rp 16.19.20.7 NMAC, 06-26-2018] 16.19.20.8 REGISTRATION REQUIREMENTS: Persons required to register: A. -

2008 Prohibited List
The World Anti-Doping Code THE 2008 PROHIBITED LIST INTERNATIONAL STANDARD The official text of the Prohibited List shall be maintained by WADA and shall be published in English and French. In the event of any conflict between the English and French versions, the English version shall prevail. This List shall come into effect on 1 January 2008 The Prohibited List 2008 September 22, 2007 THE 2008 PROHIBITED LIST WORLD ANTI-DOPING CODE Valid 1 January 2008 The use of any drug should be limited to medically justified indications SUBSTANCES AND METHODS PROHIBITED AT ALL TIMES (IN- AND OUT-OF-COMPETITION) PROHIBITED SUBSTANCES S1. ANABOLIC AGENTS Anabolic agents are prohibited. 1. Anabolic Androgenic Steroids (AAS) a. Exogenous* AAS, including: 1-androstendiol (5α-androst-1-ene-3β,17β-diol ); 1-androstendione (5α- androst-1-ene-3,17-dione); bolandiol (19-norandrostenediol); bolasterone; boldenone; boldione (androsta-1,4-diene-3,17-dione); calusterone; clostebol; danazol (17α-ethynyl-17β-hydroxyandrost-4-eno[2,3-d]isoxazole); dehydrochlormethyltestosterone (4-chloro-17β-hydroxy-17α-methylandrosta- 1,4-dien-3-one); desoxymethyltestosterone (17α-methyl-5α-androst-2-en- 17β-ol); drostanolone; ethylestrenol (19-nor-17α-pregn-4-en-17-ol); fluoxymesterone; formebolone; furazabol (17β-hydroxy-17α-methyl-5α- androstano[2,3-c]-furazan); gestrinone; 4-hydroxytestosterone (4,17β- dihydroxyandrost-4-en-3-one); mestanolone; mesterolone; metenolone; methandienone (17β-hydroxy-17α-methylandrosta-1,4-dien-3-one); methandriol; methasterone (2α, 17α-dimethyl-5α-androstane-3-one-17β-ol); -

Appendix-2Final.Pdf 663.7 KB
North West ‘Through the Gate Substance Misuse Services’ Drug Testing Project Appendix 2 – Analytical methodologies Overview Urine samples were analysed using three methodologies. The first methodology (General Screen) was designed to cover a wide range of analytes (drugs) and was used for all analytes other than the synthetic cannabinoid receptor agonists (SCRAs). The analyte coverage included a broad range of commonly prescribed drugs including over the counter medications, commonly misused drugs and metabolites of many of the compounds too. This approach provided a very powerful drug screening tool to investigate drug use/misuse before and whilst in prison. The second methodology (SCRA Screen) was specifically designed for SCRAs and targets only those compounds. This was a very sensitive methodology with a method capability of sub 100pg/ml for over 600 SCRAs and their metabolites. Both methodologies utilised full scan high resolution accurate mass LCMS technologies that allowed a non-targeted approach to data acquisition and the ability to retrospectively review data. The non-targeted approach to data acquisition effectively means that the analyte coverage of the data acquisition was unlimited. The only limiting factors were related to the chemical nature of the analyte being looked for. The analyte must extract in the sample preparation process; it must chromatograph and it must ionise under the conditions used by the mass spectrometer interface. The final limiting factor was presence in the data processing database. The subsequent study of negative MDT samples across the North West and London and the South East used a GCMS methodology for anabolic steroids in addition to the General and SCRA screens.