Appendix a Glossary of Nuclear Terms Absorber: Any Material That Stops Ionizing Radiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

Free Radical Reactions (Read Chapter 4) A
Chemistry 5.12 Spri ng 2003, Week 3 / Day 2 Handout #7: Lecture 11 Outline IX. Free Radical Reactions (Read Chapter 4) A. Chlorination of Methane (4-2) 1. Mechanism (4-3) B. Review of Thermodynamics (4-4,5) C. Review of Kinetics (4-8,9) D. Reaction-Energy Diagrams (4-10) 1. Thermodynamic Control 2. Kinetic Control 3. Hammond Postulate (4-14) 4. Multi-Step Reactions (4-11) 5. Chlorination of Methane (4-7) Suggested Problems: 4-35–37,40,43 IX. A. Radical Chlorination of Methane H heat (D) or H H C H Cl Cl H C Cl H Cl H light (hv) H H Cl Cl D or hv D or hv etc. H C Cl H C Cl H Cl Cl C Cl H Cl Cl Cl Cl Cl H H H • Why is light or heat necessary? • How fast does it go? • Does it give off or consume heat? • How fast are each of the successive reactions? • Can you control the product ratio? To answer these questions, we need to: 1. Understand the mechanism of the reaction (arrow-pushing!). 2. Use thermodynamics and kinetics to analyze the reaction. 1 1. Mechanism of Radical Chlorination of Methane (Free-Radical Chain Reaction) Free-radical chain reactions have three distinct mechanistic steps: • initiation step: generates reactive intermediate • propagation steps: reactive intermediates react with stable molecules to generate other reactive intermediates (allows chain to continue) • termination step: side-reactions that slow the reaction; usually combination of two reactive intermediates into one stable molecule Initiation Step: Cl2 absorbs energy and the bond is homolytically cleaved. -

4. Particle Generators/Accelerators
Joint innovative training and teaching/ learning program in enhancing development and transfer knowledge of application of ionizing radiation in materials processing 4. Particle Generators/Accelerators Diana Adlienė Department of Physics Kaunas University of Technolog y Joint innovative training and teaching/ learning program in enhancing development and transfer knowledge of application of ionizing radiation in materials processing This project has been funded with support from the European Commission. This publication reflects the views only of the author. Polish National Agency and the Commission cannot be held responsible for any use which may be made of the information contained therein. Date: Oct. 2017 DISCLAIMER This presentation contains some information addapted from open access education and training materials provided by IAEA TABLE OF CONTENTS 1. Introduction 2. X-ray machines 3. Particle generators/accelerators 4. Types of industrial irradiators The best accelerator in the universe… INTRODUCTION • Naturally occurring radioactive sources: – Up to 5 MeV Alpha’s (helium nuclei) – Up to 3 MeV Beta particles (electrons) • Natural sources are difficult to maintain, their applications are limited: – Chemical processing: purity, messy, and expensive; – Low intensity; – Poor geometry; – Uncontrolled energies, usually very broad Artificial sources (beams) are requested! INTRODUCTION • Beams of accelerated particles can be used to produce beams of secondary particles: Photons (x-rays, gamma-rays, visible light) are generated from beams -

1.1. Introduction the Phenomenon of Positron Annihilation Spectroscopy
PRINCIPLES OF POSITRON ANNIHILATION Chapter-1 __________________________________________________________________________________________ 1.1. Introduction The phenomenon of positron annihilation spectroscopy (PAS) has been utilized as nuclear method to probe a variety of material properties as well as to research problems in solid state physics. The field of solid state investigation with positrons started in the early fifties, when it was recognized that information could be obtained about the properties of solids by studying the annihilation of a positron and an electron as given by Dumond et al. [1] and Bendetti and Roichings [2]. In particular, the discovery of the interaction of positrons with defects in crystal solids by Mckenize et al. [3] has given a strong impetus to a further elaboration of the PAS. Currently, PAS is amongst the best nuclear methods, and its most recent developments are documented in the proceedings of the latest positron annihilation conferences [4-8]. PAS is successfully applied for the investigation of electron characteristics and defect structures present in materials, magnetic structures of solids, plastic deformation at low and high temperature, and phase transformations in alloys, semiconductors, polymers, porous material, etc. Its applications extend from advanced problems of solid state physics and materials science to industrial use. It is also widely used in chemistry, biology, and medicine (e.g. locating tumors). As the process of measurement does not mostly influence the properties of the investigated sample, PAS is a non-destructive testing approach that allows the subsequent study of a sample by other methods. As experimental equipment for many applications, PAS is commercially produced and is relatively cheap, thus, increasingly more research laboratories are using PAS for basic research, diagnostics of machine parts working in hard conditions, and for characterization of high-tech materials. -

Installation of the Cyclotron Based Clinical Neutron Therapy System in Seattle
Proceedings of the Tenth International Conference on Cyclotrons and their Applications, East Lansing, Michigan, USA INSTALLATION OF THE CYCLOTRON BASED CLINICAL NEUTRON THERAPY SYSTEM IN SEATTLE R. Risler, J. Eenmaa, J. Jacky, I. Kalet, and P. Wootton Medical Radiation Physics RC-08, University of Washington, Seattle ~ 98195, USA S. Lindbaeck Instrument AB Scanditronix, Uppsala, Sweden Sumnary radiation areas is via sliding shielding doors rather than a maze, mai nl y to save space. For the same reason A cyclotron facility has been built for cancer a single door is used to alternately close one or the treatment with fast neutrons. 50.5 MeV protons from a other therapy room. All power supplies and the control conventional, positive ion cyclotron are used to b0m computer are located on the second floor, above the bard a semi-thick Beryllium target located 150 am from maintenance area. The cooler room contains a heat the treatment site. Two treatment rooms are available, exchanger and other refrigeration equipment. Not shown one with a fixed horizontal beam and one with an iso in the diagram is the cooling tower located in another centric gantry capable of 360 degree rotation. In part of the building. Also not shown is the hot lab addition 33 51 MeV protons and 16.5 - 25.5 MeV now under construction an an area adjacent to the deuterons are generated for isotope production inside cyclotron vault. It will be used to process radio the cyclotron vault. The control computer is also used isotopes produced at a target station in the vault. to record and verify treatment parameters for indi vidual patients and to set up and monitor the actual radiation treatment. -

00307399.Pdf
+ ASI&’rrrativc ActioIs/Eq51dS&MtSUdtyErrqsbyer < This work was supported by the National Cancer Institute, Division of Research Resources and Centers, Department of Health, Education, and Welfare. Edited by Ixruise Taylor, AT Division. DISCLAMER This report was prepared as an account of work sponsored by assagency of the Uruted States &rvcrrrment. Neither the United States Ciovernanerstnor any agency thereof, nor asryof their employru, makes any *anty, express or irnpfied, or assumesany legal liability or responsibility for the accuracy, completeness, or usefulnessof arsyirsfornsation, apparatus, product, or processdisclosed, or represents that its use would not infringe privately owned rights. References herein to any specitlc comnserciat product. process, or aer+ce by trade name, trademark, rnamafacmrer, or otherwise, does not necessady constitute or imply ita errdorsement, recommendation, or favoring by the Urdted States Government or any agency thersof. llte view and opinions of authors expressed herein do not nemsas-ity stare or reflect those of the United States Government or any agency thereof. LA-9144-MS UC-28 and UC-48 Issued: February 1982 . ● / A Linear Accelerator for Radioisotope Production L.D. Hansborough R. W. Harem J. E. Stovall i !-=-—- ‘ - I I . -.... J“’”’ .“ Los Alamos National Laboratory ~~~~la~~s Lo.Alamo.,New.exi..875.~ A LINEAR ACCELERATOR FOR RADIOISOTOPE PRODUCTION by L. D. Hansborough, R. W. Harem, and J. E. Stovall ABSTRACT A 200- to 500-uA source of 70- to 90-MeV protons would be a valuable asset to the nuclear medicine program. A linear accelerator (linac) can achieve this performance, and it can be extended to even higher energies and currents. Variable energy and current options are available. -

22 Thesis Cyclotron Design and Construction Design and Construction of a Cyclotron Capable of Accelerating Protons
22 Thesis Cyclotron Design and Construction Design and Construction of a Cyclotron Capable of Accelerating Protons to 2 MeV by Leslie Dewan Submitted to the Department of Nuclear Science and Engineering in Partial Fulfillment of the Requirements for the Degree of Bachelor of Science in Nuclear Science and Engineering at the Massachusetts Institute of Technology June 2007 © 2007 Leslie Dewan All rights reserved The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or hereafter created. Signature of Author: Leslie Dewan Department of Nuclear Science and Engineering May 16, 2007 Certified by: David G. Cory Professor of Nuclear Science and Engineering Thesis Supervisor Accepted by: David G. Cory Professor of Nuclear Science and Engineering Chairman, NSE Committee for Undergraduate Students Leslie Dewan 1 of 23 5/16/07 22 Thesis Cyclotron Design and Construction Design and Construction of a Cyclotron Capable of Accelerating Protons to 2 MeV by Leslie Dewan Submitted to the Department of Nuclear Science and Engineering on May 16, 2007 in Partial Fulfillment of the Requirements for the Degree of Bachelor of Science in Nuclear Science and Engineering ABSTRACT This thesis describes the design and construction of a cyclotron capable of accelerating protons to 2 MeV. A cyclotron is a charged particle accelerator that uses a magnetic field to confine particles to a spiral flight path in a vacuum chamber. An applied electrical field accelerates these particles to high energies, typically on the order of mega-electron volts. -

25 Geometric Optics
CHAPTER 25 | GEOMETRIC OPTICS 887 25 GEOMETRIC OPTICS Figure 25.1 Image seen as a result of reflection of light on a plane smooth surface. (credit: NASA Goddard Photo and Video, via Flickr) Learning Objectives 25.1. The Ray Aspect of Light • List the ways by which light travels from a source to another location. 25.2. The Law of Reflection • Explain reflection of light from polished and rough surfaces. 25.3. The Law of Refraction • Determine the index of refraction, given the speed of light in a medium. 25.4. Total Internal Reflection • Explain the phenomenon of total internal reflection. • Describe the workings and uses of fiber optics. • Analyze the reason for the sparkle of diamonds. 25.5. Dispersion: The Rainbow and Prisms • Explain the phenomenon of dispersion and discuss its advantages and disadvantages. 25.6. Image Formation by Lenses • List the rules for ray tracking for thin lenses. • Illustrate the formation of images using the technique of ray tracking. • Determine power of a lens given the focal length. 25.7. Image Formation by Mirrors • Illustrate image formation in a flat mirror. • Explain with ray diagrams the formation of an image using spherical mirrors. • Determine focal length and magnification given radius of curvature, distance of object and image. Introduction to Geometric Optics Geometric Optics Light from this page or screen is formed into an image by the lens of your eye, much as the lens of the camera that made this photograph. Mirrors, like lenses, can also form images that in turn are captured by your eye. 888 CHAPTER 25 | GEOMETRIC OPTICS Our lives are filled with light. -

How Do Radioactive Materials Move Through the Environment to People?
5. How Do Radioactive Materials Move Through the Environment to People? aturally occurring radioactive materials Radionuclides can be removed from the air in Nare present in our environment and in several ways. Particles settle out of the our bodies. We are, therefore, continuously atmosphere if air currents cannot keep them exposed to radiation from radioactive atoms suspended. Rain or snow can also remove (radionuclides). Radionuclides released to them. the environment as a result of human When these particles are removed from the activities add to that exposure. atmosphere, they may land in water, on soil, or Radiation is energy emitted when a on the surfaces of living and non-living things. radionuclide decays. It can affect living tissue The particles may return to the atmosphere by only when the energy is absorbed in that resuspension, which occurs when wind or tissue. Radionuclides can be hazardous to some other natural or human activity living tissue when they are inside an organism generates clouds of dust containing radionu- where radiation released can be immediately clides. absorbed. They may also be hazardous when they are outside of the organism but close ➤ Water enough for some radiation to be absorbed by Radionuclides can come into contact with the tissue. water in several ways. They may be deposited Radionuclides move through the environ- from the air (as described above). They may ment and into the body through many also be released to the water from the ground different pathways. Understanding these through erosion, seepage, or human activities pathways makes it possible to take actions to such as mining or release of radioactive block or avoid exposure to radiation. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Glossary of Scientific Terms in the Mystery of Matter
GLOSSARY OF SCIENTIFIC TERMS IN THE MYSTERY OF MATTER Term Definition Section acid A substance that has a pH of less than 7 and that can react with 1 metals and other substances. air The mixture of oxygen, nitrogen, and other gasses that is consistently 1 present around us. alchemist A person who practices a form of chemistry from the Middle Ages 1 that was concerned with transforming various metals into gold. Alchemy A type of science and philosophy from the Middle Ages that 1 attempted to perform unusual experiments, taking something ordinary and turning it into something extraordinary. alkali metals Any of a group of soft metallic elements that form alkali solutions 3 when they combine with water. They include lithium, sodium, potassium, rubidium, cesium, and francium. alkaline earth Any of a group of metallic elements that includes beryllium, 3 metals magnesium, calcium, strontium, barium, and radium. alpha particle A positively charged particle, indistinguishable from a helium atom 5, 6 nucleus and consisting of two protons and two neutrons. alpha decay A type of radioactive decay in which a nucleus emits 6 an alpha particle. aplastic anemia A disorder of the bone marrow that results in too few blood cells. 4 apothecary The person in a pharmacy who distributes medicine. 1 atom The smallest component of an element that shares the chemical 1, 2, 3, 4, 5, 6 properties of the element and contains a nucleus with neutrons, protons, and electrons. atomic bomb A bomb whose explosive force comes from a chain reaction based on 6 nuclear fission. atomic number The number of protons in the nucleus of an atom. -
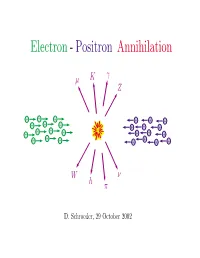
Electron - Positron Annihilation
Electron - Positron Annihilation γ µ K Z − − − + + + − − − + + + − − − − + + + − − − + + + + W ν h π D. Schroeder, 29 October 2002 OUTLINE • Electron-positron storage rings • Detectors • Reaction examples e+e− −→ e+e− [Inventory of known particles] e+e− −→ µ+µ− e+e− −→ q q¯ e+e− −→ W +W − • The future:Linear colliders Electron-Positron Colliders Hamburg Novosibirsk 11 GeV 12 GeV 47 GeV Geneva 200 GeV Ithaca Tokyo Stanford 12 GeV 64 GeV 8 GeV 12 GeV 30 GeV 100 GeV Beijing 12 GeV 4 GeV Size (R) and Cost ($) of an e+e− Storage Ring βE4 $=αR + ( E = beam energy) R d $ βE4 Find minimum $: 0= = α − dR R2 β =⇒ R = E2, $=2 αβ E2 α SPEAR: E = 8 GeV, R = 40 m, $ = 5 million LEP: E = 200 GeV, R = 4.3 km, $ = 1 billion + − + − Example 1: e e −→ e e e− total momentum = 0 θ − total energy = 2E e e+ Probability(E,θ)=? e+ E-dependence follows from dimensional analysis: density = ρ− A density = ρ+ − + × 2 Probability = (ρ− ρ+ − + A) (something with units of length ) ¯h ¯hc When E m , the only relevant length is = e p E 1 =⇒ Probability ∝ E2 at SPEAR Augustin, et al., PRL 34, 233 (1975) 6000 5000 4000 Ecm = 4.8 GeV 3000 2000 Number of Counts 1000 Theory 0 −0.8 −0.40 0.40.8 cos θ Prediction for e+e− −→ e+e− event rate (H. J. Bhabha, 1935): event dσ e4 1 + cos4 θ 2 cos4 θ 1 + cos2 θ ∝ = 2 − 2 + rate dΩ 32π2E2 4 θ 2 θ 2 cm sin 2 sin 2 Interpretation of Bhabha’sformula (R.