Apatelodes Torrefacta (J.E. Smith, 1797) (Lepidoptera: Apatelodidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Butterflies and Moths of Gwinnett County, Georgia, United States
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail -

Proceedings of the United States National Museum
DESCRIPTIONS OF NEW SPECIES AND GENERA OF LEPIDOPTERA, CHIEFLY' FIIOM MEXICO. By IIarrisox G. Dyar, Custodian of Lcpidoptera, United States National Museum. TJie followint; apparently undescribcd species have mostly been received from Mr. Roberto Miiller, of Mexico City, for identification. I have been assisted in placing some of the species by Sir George F. Ilampson and Mr. William Schaus. Their assistance is specially acknowledged under each heading. All the species are from Mexico excei)t in one famil}', the Cochlidiidae, where species from Costa Rica and Brazil are described. Superfamily PAPILIONOIDEA. Family SATYRID^. Genus EUPTYCHIA Hubner. EUPTYCHIA PERTEPIDA, new species. Dark gray; a reddish shade over the middle of the fore wing, espe- cially marked along the median vein and the bases of veins 3 and 4; a diffused band of erect scales across the disk beyond the median vein, cut by the reddish veins. Hind ^ving with the diffused reddish shade outwardly; two elongated blackish spots on the margin between veins 3 to 5. Beneath the fore wings are reddish on the lower half; two brown lines cross the disk, and there is a row of submarginal lunate dusky spots. Ilind wing brown-gray, the two median lines wavy and irregular, with a faint similar subbasal line, the outermost line followed by a bright reddish shade. A submarginal row of silvery scaling in a waved and broken line, crossing two velvet}' black oval spots on the margin, on which the silver forms irregularl}' geminate spots. Expanse, 35 mm. Female similar, but the whole discal area of fore whig overspread willi bright brownish red, the Imes of the underside slightly indicated, the SOX mark absent. -

CHECKLIST of WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea)
WISCONSIN ENTOMOLOGICAL SOCIETY SPECIAL PUBLICATION No. 6 JUNE 2018 CHECKLIST OF WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea) Leslie A. Ferge,1 George J. Balogh2 and Kyle E. Johnson3 ABSTRACT A total of 1284 species representing the thirteen families comprising the present checklist have been documented in Wisconsin, including 293 species of Geometridae, 252 species of Erebidae and 584 species of Noctuidae. Distributions are summarized using the six major natural divisions of Wisconsin; adult flight periods and statuses within the state are also reported. Examples of Wisconsin’s diverse native habitat types in each of the natural divisions have been systematically inventoried, and species associated with specialized habitats such as peatland, prairie, barrens and dunes are listed. INTRODUCTION This list is an updated version of the Wisconsin moth checklist by Ferge & Balogh (2000). A considerable amount of new information from has been accumulated in the 18 years since that initial publication. Over sixty species have been added, bringing the total to 1284 in the thirteen families comprising this checklist. These families are estimated to comprise approximately one-half of the state’s total moth fauna. Historical records of Wisconsin moths are relatively meager. Checklists including Wisconsin moths were compiled by Hoy (1883), Rauterberg (1900), Fernekes (1906) and Muttkowski (1907). Hoy's list was restricted to Racine County, the others to Milwaukee County. Records from these publications are of historical interest, but unfortunately few verifiable voucher specimens exist. Unverifiable identifications and minimal label data associated with older museum specimens limit the usefulness of this information. Covell (1970) compiled records of 222 Geometridae species, based on his examination of specimens representing at least 30 counties. -

Influence of Habitat and Bat Activity on Moth Community Composition and Seasonal Phenology Across Habitat Types
INFLUENCE OF HABITAT AND BAT ACTIVITY ON MOTH COMMUNITY COMPOSITION AND SEASONAL PHENOLOGY ACROSS HABITAT TYPES BY MATTHEW SAFFORD THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Science in Entomology in the Graduate College of the University of Illinois at Urbana-Champaign, 2018 Urbana, Illinois Advisor: Assistant Professor Alexandra Harmon-Threatt, Chair and Director of Research ABSTRACT Understanding the factors that influence moth diversity and abundance is important for monitoring moth biodiversity and developing conservation strategies. Studies of moth habitat use have primarily focused on access to host plants used by specific moth species. How vegetation structure influences moth communities within and between habitats and mediates the activity of insectivorous bats is understudied. Previous research into the impact of bat activity on moths has primarily focused on interactions in a single habitat type or a single moth species of interest, leaving a large knowledge gap on how habitat structure and bat activity influence the composition of moth communities across habitat types. I conducted monthly surveys at sites in two habitat types, restoration prairie and forest. Moths were collected using black light bucket traps and identified to species. Bat echolocation calls were recorded using ultrasonic detectors and classified into phonic groups to understand how moth community responds to the presence of these predators. Plant diversity and habitat structure variables, including tree diameter at breast height, ground cover, and vegetation height were measured during summer surveys to document how differences in habitat structure between and within habitats influences moth diversity. I found that moth communities vary significantly between habitat types. -

Phylogenomics Reveals Major Diversification Rate Shifts in The
bioRxiv preprint doi: https://doi.org/10.1101/517995; this version posted January 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Phylogenomics reveals major diversification rate shifts in the evolution of silk moths and 2 relatives 3 4 Hamilton CA1,2*, St Laurent RA1, Dexter, K1, Kitching IJ3, Breinholt JW1,4, Zwick A5, Timmermans 5 MJTN6, Barber JR7, Kawahara AY1* 6 7 Institutional Affiliations: 8 1Florida Museum of Natural History, University of Florida, Gainesville, FL 32611 USA 9 2Department of Entomology, Plant Pathology, & Nematology, University of Idaho, Moscow, ID 10 83844 USA 11 3Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK 12 4RAPiD Genomics, 747 SW 2nd Avenue #314, Gainesville, FL 32601. USA 13 5Australian National Insect Collection, CSIRO, Clunies Ross St, Acton, ACT 2601, Canberra, 14 Australia 15 6Department of Natural Sciences, Middlesex University, The Burroughs, London NW4 4BT, UK 16 7Department of Biological Sciences, Boise State University, Boise, ID 83725, USA 17 *Correspondence: [email protected] (CAH) or [email protected] (AYK) 18 19 20 Abstract 21 The silkmoths and their relatives (Bombycoidea) are an ecologically and taxonomically 22 diverse superfamily that includes some of the most charismatic species of all the Lepidoptera. 23 Despite displaying some of the most spectacular forms and ecological traits among insects, 24 relatively little attention has been given to understanding their evolution and the drivers of 25 their diversity. -

Papilio (New Series) # 25 2016 Issn 2372-9449
PAPILIO (NEW SERIES) # 25 2016 ISSN 2372-9449 ERNEST J. OSLAR, 1858-1944: HIS TRAVEL AND COLLECTION ITINERARY, AND HIS BUTTERFLIES by James A. Scott, Ph.D. in entomology University of California Berkeley, 1972 (e-mail: [email protected]) Abstract. Ernest John Oslar collected more than 50,000 butterflies and moths and other insects and sold them to many taxonomists and museums throughout the world. This paper attempts to determine his travels in America to collect those specimens, by using data from labeled specimens (most in his remaining collection but some from published papers) plus information from correspondence etc. and a few small field diaries preserved by his descendants. The butterfly specimens and their localities/dates in his collection in the C. P. Gillette Museum (Colorado State University, Fort Collins, Colorado) are detailed. This information will help determine the possible collection locations of Oslar specimens that lack accurate collection data. Many more biographical details of Oslar are revealed, and the 26 insects named for Oslar are detailed. Introduction The last collection of Ernest J. Oslar, ~2159 papered butterfly specimens and several moths, was found in the C. P. Gillette Museum, Colorado State University, Fort Collins, Colorado by Paul A. Opler, providing the opportunity to study his travels and collections. Scott & Fisher (2014) documented specimens sent by Ernest J. Oslar of about 100 Argynnis (Speyeria) nokomis nokomis Edwards labeled from the San Juan Mts. and Hall Valley of Colorado, which were collected by Wilmatte Cockerell at Beulah New Mexico, and documented Oslar’s specimens of Oeneis alberta oslari Skinner labeled from Deer Creek Canyon, [Jefferson County] Colorado, September 25, 1909, which were collected in South Park, Park Co. -

Impacts of Native and Non-Native Plants on Urban Insect Communities: Are Native Plants Better Than Non-Natives?
Impacts of Native and Non-native plants on Urban Insect Communities: Are Native Plants Better than Non-natives? by Carl Scott Clem A thesis submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Master of Science Auburn, Alabama December 12, 2015 Key Words: native plants, non-native plants, caterpillars, natural enemies, associational interactions, congeneric plants Copyright 2015 by Carl Scott Clem Approved by David Held, Chair, Associate Professor: Department of Entomology and Plant Pathology Charles Ray, Research Fellow: Department of Entomology and Plant Pathology Debbie Folkerts, Assistant Professor: Department of Biological Sciences Robert Boyd, Professor: Department of Biological Sciences Abstract With continued suburban expansion in the southeastern United States, it is increasingly important to understand urbanization and its impacts on sustainability and natural ecosystems. Expansion of suburbia is often coupled with replacement of native plants by alien ornamental plants such as crepe myrtle, Bradford pear, and Japanese maple. Two projects were conducted for this thesis. The purpose of the first project (Chapter 2) was to conduct an analysis of existing larval Lepidoptera and Symphyta hostplant records in the southeastern United States, comparing their species richness on common native and alien woody plants. We found that, in most cases, native plants support more species of eruciform larvae compared to aliens. Alien congener plant species (those in the same genus as native species) supported more species of larvae than alien, non-congeners. Most of the larvae that feed on alien plants are generalist species. However, most of the specialist species feeding on alien plants use congeners of native plants, providing evidence of a spillover, or false spillover, effect. -

Alternative Moth-Eye Nanostructures: Antireflective Properties and Composition of Dimpled Corneal Nanocoatings in Silk-Moth Ancestors
Research Collection Journal Article Alternative moth-eye nanostructures: antireflective properties and composition of dimpled corneal nanocoatings in silk-moth ancestors Author(s): Kryuchkov, Mikhail; Lehmann, Jannis; Schaab, Jakob; Cherepanov, Vsevolod; Blagodatski, Artem; Fiebig, Manfred; Katanaev, Vladimir L. Publication Date: 2017 Permanent Link: https://doi.org/10.3929/ethz-b-000191061 Originally published in: Journal of Nanobiotechnology 15, http://doi.org/10.1186/s12951-017-0297-y Rights / License: Creative Commons Attribution 4.0 International This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Kryuchkov et al. J Nanobiotechnol (2017) 15:61 DOI 10.1186/s12951-017-0297-y Journal of Nanobiotechnology SHORT COMMUNICATION Open Access Alternative moth‑eye nanostructures: antirefective properties and composition of dimpled corneal nanocoatings in silk‑moth ancestors Mikhail Kryuchkov1, Jannis Lehmann2, Jakob Schaab2, Vsevolod Cherepanov3, Artem Blagodatski1,3, Manfred Fiebig2 and Vladimir L. Katanaev1,3* Abstract Moth-eye nanostructures are a well-known example of biological antirefective surfaces formed by pseudoregular arrays of nipples and are often used as a template for biomimetic materials. Here, we provide morphological charac- terization of corneal nanostructures of moths from the Bombycidae family, including strains of domesticated Bombyx mori silk-moth, its wild ancestor Bombyx mandarina, and a more distantly related Apatelodes torrefacta. We fnd high diversifcation of the nanostructures and strong antirefective properties they provide. Curiously, the nano-dimple pat- tern of B. mandarina is found to reduce refectance as efciently as the nanopillars of A. torrefacta. Access to genome sequence of Bombyx further permitted us to pinpoint corneal proteins, likely contributing to formation of the antire- fective nanocoatings. -

8 March 2013, 381 P
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/273257107 Mason, P. G., D. R. Gillespie & C. Vincent (Eds.) 2013. Proceedings of the Fourth International Symposium on Biological Control of Arthropods. Pucón, Chile, 4-8 March 2013, 381 p. CONFERENCE PAPER · MARCH 2013 DOWNLOADS VIEWS 626 123 3 AUTHORS, INCLUDING: Peter Mason Charles Vincent Agriculture and Agri-Food Canada Agriculture and Agri-Food Canada 96 PUBLICATIONS 738 CITATIONS 239 PUBLICATIONS 1,902 CITATIONS SEE PROFILE SEE PROFILE Available from: Charles Vincent Retrieved on: 13 August 2015 The correct citation of this work is: Peter G. Mason, David R. Gillespie and Charles Vincent (Eds.). 2013. Proceedings of the 4th International Symposium on Biological Control of Arthropods. Pucón, Chile, 4-8 March 2013, 380 p. Proceedings of the 4th INTERNATIONAL SYMPOSIUM ON BIOLOGICAL CONTROL OF ARTHROPODS Pucón, Chile March 4-8, 2013 Peter G. Mason, David R. Gillespie and Charles Vincent (Eds.) 4th INTERNATIONAL SYMPOSIUM ON BIOLOGICAL CONTROL OF ARTHROPODS Pucón, Chile, March 4-8, 2013 PREFACE The Fourth International Symposium on Biological Control of Arthropods, held in Pucón – Chile, continues the series of international symposia on the biological control of arthropods organized every four years. The first meeting was in Hawaii – USA during January 2002, followed by the Davos - Switzerland meeting during September 2005, and the Christchurch – New Zealand meeting during February 2009. The goal of these symposia is to create a forum where biological control researchers and practitioners can meet and exchange information, to promote discussions of up to date issues affecting biological control, particularly pertaining to the use of parasitoids and predators as biological control agents. -

Insecta: Lepidoptera)
Biodiversity Data Journal 6: e22236 doi: 10.3897/BDJ.6.e22236 Taxonomic Paper A global checklist of the Bombycoidea (Insecta: Lepidoptera) Ian J Kitching‡, Rodolphe Rougerie§, Andreas Zwick |, Chris A Hamilton¶, Ryan A St Laurent¶, Stefan Naumann#, Liliana Ballesteros Mejia§,¤, Akito Y Kawahara¶ ‡ Natural History Museum, London, United Kingdom § Muséum national d’Histoire naturelle, Sorbonne Université, Institut de Systématique, Evolution, Biodiversité (ISYEB), UMR 7205 – CNRS, MNHN, UPMC, EPHE, Paris, France | CSIRO - Australian National Insect Collection, Canberra, Australia ¶ Florida Museum of Natural History, University of Florida, Gainesville, United States of America # Hochkirchstrasse 71, Berlin, Germany ¤ CESAB, Centre de Synthèse et d'Analyse sur la Biodiversité, Aix-en-Provence, France Corresponding author: Ian J Kitching ([email protected]), Rodolphe Rougerie ([email protected]) Academic editor: Yasen Mutafchiev Received: 13 Nov 2017 | Accepted: 08 Feb 2018 | Published: 12 Feb 2018 Citation: Kitching I, Rougerie R, Zwick A, Hamilton C, St Laurent R, Naumann S, Ballesteros Mejia L, Kawahara A (2018) A global checklist of the Bombycoidea (Insecta: Lepidoptera). Biodiversity Data Journal 6: e22236. https://doi.org/10.3897/BDJ.6.e22236 ZooBank: urn:lsid:zoobank.org:pub:937DDBF7-10F3-4700-B188-227F33800216 Abstract Background Bombycoidea is an ecologically diverse and speciose superfamily of Lepidoptera. The superfamily includes many model organisms, but the taxonomy and classification of the superfamily has remained largely in disarray. Here we present a global checklist of Bombycoidea. Following Zwick (2008) and Zwick et al. (2011), ten families are recognized: Anthelidae, Apatelodidae, Bombycidae, Brahmaeidae, Carthaeidae, Endromidae, Eupterotidae, Phiditiidae, Saturniidae and Sphingidae. The former families Lemoniidae and Mirinidae are included within Brahmaeidae and Endromidae respectively. -
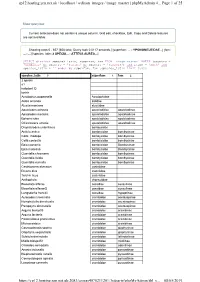
Page 1 of 25 Cp12.Hosting.Zen.Net.Uk / Localhost / Wdixon Images / Image Master | Phpmyadmin 4... 08/05/2019
cp12.hosting.zen.net.uk / localhost / wdixon_images / image_master | phpMyAdmin 4... Page 1 of 25 Show query box Current selection does not contain a unique column. Grid edit, checkbox, Edit, Copy and Delete features are not available. Showing rows 0 - 657 (658 total, Query took 0.0117 seconds.) [superfam: ... - YPONOMEUTIDAE...] [fam: ... - ...] [species_latin: 2 SPECIS... - ATTEVA AUREA...] SELECT distinct species_latin, superfam, fam FROM `image_master` WHERE (country = 'Honduras' or country = 'Panama' or country = 'Pan2018') and itype = 'moth' and species_latin > '' order by superfam, fam ,species_latin limit 0,850 species_latin 3 superfam 1 fam 2 2 specis a1 notodont Q tortrix Acrolophus popeanella Acrolophidae Aidos amanda aididae Alucita montana alucitidae Apatelodes adrastia apatelodidae apatelodinae Apatelodes merlona apatelodidae apatelodinae Ephoria lybia apatelodidae apatelodinae Olceclostera amoria apatelodidae apatelodinae Drepatelodes umbrillinea bombycidae Anticla antica bombycidae bombycinae Colla rhodope bombycidae bombycinae Colla coelestis bombycidae bombycinae Epia casnonia bombycidae Bombycinae Epia muscosa bombycidae Bombycinae Quentalia chromana bombycidae bombycinae Quentalia lividia bombycidae bombycinae Quentalia numalia bombycidae bombycinae Castniomera atymnius castniidae Divana diva castniidae Telchin licus castniidae Anthophyla choreutidae Biocellata alfarae cossidae cossulinae Biocellata alfaraeQ cossidae cossulinae Langsdorfia franckii cossidae hypoptinae Aulacodes traversalis crambidae acentropinae Nymphuliella