The Nic Cluster from Pseudomonas Putida KT2440
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metaproteogenomic Insights Beyond Bacterial Response to Naphthalene
ORIGINAL ARTICLE ISME Journal – Original article Metaproteogenomic insights beyond bacterial response to 5 naphthalene exposure and bio-stimulation María-Eugenia Guazzaroni, Florian-Alexander Herbst, Iván Lores, Javier Tamames, Ana Isabel Peláez, Nieves López-Cortés, María Alcaide, Mercedes V. del Pozo, José María Vieites, Martin von Bergen, José Luis R. Gallego, Rafael Bargiela, Arantxa López-López, Dietmar H. Pieper, Ramón Rosselló-Móra, Jesús Sánchez, Jana Seifert and Manuel Ferrer 10 Supporting Online Material includes Text (Supporting Materials and Methods) Tables S1 to S9 Figures S1 to S7 1 SUPPORTING TEXT Supporting Materials and Methods Soil characterisation Soil pH was measured in a suspension of soil and water (1:2.5) with a glass electrode, and 5 electrical conductivity was measured in the same extract (diluted 1:5). Primary soil characteristics were determined using standard techniques, such as dichromate oxidation (organic matter content), the Kjeldahl method (nitrogen content), the Olsen method (phosphorus content) and a Bernard calcimeter (carbonate content). The Bouyoucos Densimetry method was used to establish textural data. Exchangeable cations (Ca, Mg, K and 10 Na) extracted with 1 M NH 4Cl and exchangeable aluminium extracted with 1 M KCl were determined using atomic absorption/emission spectrophotometry with an AA200 PerkinElmer analyser. The effective cation exchange capacity (ECEC) was calculated as the sum of the values of the last two measurements (sum of the exchangeable cations and the exchangeable Al). Analyses were performed immediately after sampling. 15 Hydrocarbon analysis Extraction (5 g of sample N and Nbs) was performed with dichloromethane:acetone (1:1) using a Soxtherm extraction apparatus (Gerhardt GmbH & Co. -

Downloaded 10/5/2021 9:43:05 AM
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue Aromatic side-chain flips orchestrate the conformational sampling of functional loops in Cite this: Chem. Sci.,2021,12,9318 † All publication charges for this article human histone deacetylase 8 have been paid for by the Royal Society a a bcd of Chemistry Vaibhav Kumar Shukla, ‡ Lucas Siemons, ‡ Francesco L. Gervasio and D. Flemming Hansen *a Human histone deacetylase 8 (HDAC8) is a key hydrolase in gene regulation and an important drug-target. High-resolution structures of HDAC8 in complex with substrates or inhibitors are available, which have provided insights into the bound state of HDAC8 and its function. Here, using long all-atom unbiased molecular dynamics simulations and Markov state modelling, we show a strong correlation between the conformation of aromatic side chains near the active site and opening and closing of the surrounding functional loops of HDAC8. We also investigated two mutants known to allosterically downregulate the enzymatic activity of HDAC8. Based on experimental data, we hypothesise that I19S-HDAC8 is unable to Received 6th April 2021 Creative Commons Attribution 3.0 Unported Licence. release the product, whereas both product release and substrate binding are impaired in the S39E- Accepted 27th May 2021 HDAC8 mutant. The presented results deliver detailed insights into the functional dynamics of HDAC8 DOI: 10.1039/d1sc01929e and provide a mechanism for the substantial downregulation caused by allosteric mutations, including rsc.li/chemical-science a disease causing one. Introduction II (HDAC-4, -5, -6, -7, -9, and HDAC10), and class III (SIRT1-7) have sequence similarity to yeast Rpd3, Hda1, and Sir2, Acetylation of lysine side chains occurs as a co-translation or respectively, whereas class IV (HDAC11) shares sequence simi- 2 This article is licensed under a post-translational modication of proteins and was rst iden- larity with both class I and II proteins. -

Characterization of the 4-Carboxy-2-Hydroxymuconate
Characterization of the 4-carboxy-2-hydroxymuconate hydratase and the 4-carboxy-4-hydroxy-2-oxoadipate/4-hydroxy-4-methyl-2-oxoglutarate aldolase from Pseudomonas putida by Scott Mazurkewich A Thesis presented to The University of Guelph In partial fulfilment of requirements for the degree of Doctor of Philosophy in Molecular and Cellular Biology Guelph, Ontario, Canada © Scott Mazurkewich, May, 2016 ABSTRACT CHARACTERIZATION OF THE 4-CARBOXY-2-HYDROXYMUCONATE HYDRATASE AND THE 4-CARBOXY-4-HYDROXY-2-OXOADIPATE/4-HYDROXY-4- METHYL-2-OXOGLUTARATE ALDOLASE FROM PSEUDOMONAS PUTIDA Scott Mazurkewich Advisor: University of Guelph, 2016 Dr. Stephen Y.K. G. Seah The protocatechuate and gallate 4,5-cleavage pathways are important bacterial catabolic pathways for environmental carbon cycling and xenobiotic remediation. This thesis focuses on the characterization of the 4-carboxy-2-hydroxymuconate (CHM) hydratase and the 4-hydroxy- 4-methyl-2-oxoglutarate (HMG)/4-carboxy-4-hydroxy-2-oxoadipate (CHA) aldolase which are the last two steps of the protocatechuate and gallate 4,5-cleavage pathways. HMG/CHA aldolases are class II pyruvate aldolases that have structural similarity to a group of proteins termed Regulators of RNase E activity A (RraA). The Escherichia coli RraA (EcRraA) binds to the regulatory domain of RNase E, inhibiting ribonuclease activity. Sequence and kinetic analyses of homologous RraA-like proteins identified minimal motifs, either a D- X20-R-D or a G-X20-R-D-X2-E/D motif, required for metal binding and aldolase activity amongst homologs. The EcRraA, which lacked sequence conservation to either motif, lacked detectable C-C lyase activity. -
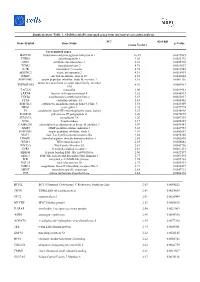
Supplementary Table 1. All Differentially Expressed Genes from Microarray Screening Analysis
Supplementary Table 1. All differentially expressed genes from microarray screening analysis. FCa (Gal-KD Gene Symbol Gene Name p-Value versus Vector) Up-regulated genes HAPLN1 hyaluronan and proteoglycan link protein 1 10.49 0.0027085 THBS1 thrombospondin 1 9.20 0.0022192 ODC1 ornithine decarboxylase 1 6.38 0.0055776 TGM2 transglutaminase 2 4.76 0.0015627 IL7R interleukin 7 receptor 4.75 0.0017245 SERINC2 serine incorporator 2 4.51 0.0014919 ITM2C integral membrane protein 2C 4.32 0.0044644 SERPINB7 serpin peptidase inhibitor, clade B, member 7 4.18 0.0081136 tumor necrosis factor receptor superfamily, member TNFRSF10D 4.01 0.0085561 10d TAGLN transgelin 3.90 0.0099963 LRRN4 leucine rich repeat neuronal 4 3.82 0.0046513 TGFB2 transforming growth factor beta 2 3.51 0.0035017 CPA4 carboxypeptidase A4 3.43 0.0008452 EPB41L3 erythrocyte membrane protein band 4.1-like 3 3.34 0.0025309 NRG1 neuregulin 1 3.28 0.0079724 F3 coagulation factor III (thromboplastin, tissue factor) 3.27 0.0038968 POLR3G polymerase III polypeptide G 3.26 0.0070675 SEMA7A semaphorin 7A 3.20 0.0087335 NT5E 5-nucleotidase 3.17 0.0036353 CAMK2N1 calmodulin-dependent protein kinase II inhibitor 1 3.07 0.0090141 TIMP3 TIMP metallopeptidase inhibitor 3 3.03 0.0047953 SERPINE1 serpin peptidase inhibitor, clade E 2.97 0.0053652 MALL mal, T-cell differentiation protein-like 2.88 0.0078205 DDAH1 dimethylarginine dimethylaminohydrolase 1 2.86 0.0002895 WDR3 WD repeat domain 3 2.85 0.0058842 WNT5A Wnt Family Member 5A 2.81 0.0043796 GPR1 G protein-coupled receptor 1 2.81 0.0021313 -

WO 2019/079361 Al 25 April 2019 (25.04.2019) W 1P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2019/079361 Al 25 April 2019 (25.04.2019) W 1P O PCT (51) International Patent Classification: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, C12Q 1/68 (2018.01) A61P 31/18 (2006.01) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, C12Q 1/70 (2006.01) HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (21) International Application Number: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, PCT/US2018/056167 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (22) International Filing Date: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 16 October 2018 (16. 10.2018) TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (26) Publication Language: English GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, (30) Priority Data: UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, 62/573,025 16 October 2017 (16. 10.2017) US TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, ΓΕ , IS, IT, LT, LU, LV, (71) Applicant: MASSACHUSETTS INSTITUTE OF MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, TECHNOLOGY [US/US]; 77 Massachusetts Avenue, TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, Cambridge, Massachusetts 02139 (US). -

Biochemical Characterization and Comparison of Aspartylglucosaminidases Secreted in Venom of the Parasitoid Wasps Asobara Tabida and Leptopilina Heterotoma
RESEARCH ARTICLE Biochemical characterization and comparison of aspartylglucosaminidases secreted in venom of the parasitoid wasps Asobara tabida and Leptopilina heterotoma Quentin Coulette1, SeÂverine Lemauf2, Dominique Colinet2, Geneviève PreÂvost1, Caroline Anselme1, Marylène Poirie 2, Jean-Luc Gatti2* a1111111111 1 Unite ªEcologie et Dynamique des Systèmes AnthropiseÂsº (EDYSAN, FRE 3498 CNRS-UPJV), Universite de Picardie Jules Verne, Amiens, France, 2 Universite CoÃte d'Azur, INRA, CNRS, ISA, Sophia Antipolis, a1111111111 France a1111111111 a1111111111 * [email protected] a1111111111 Abstract Aspartylglucosaminidase (AGA) is a low-abundance intracellular enzyme that plays a key OPEN ACCESS role in the last stage of glycoproteins degradation, and whose deficiency leads to human Citation: Coulette Q, Lemauf S, Colinet D, PreÂvost aspartylglucosaminuria, a lysosomal storage disease. Surprisingly, high amounts of AGA- G, Anselme C, Poirie M, et al. (2017) Biochemical characterization and comparison of like proteins are secreted in the venom of two phylogenetically distant hymenopteran para- aspartylglucosaminidases secreted in venom of the sitoid wasp species, Asobara tabida (Braconidae) and Leptopilina heterotoma (Cynipidae). parasitoid wasps Asobara tabida and Leptopilina These venom AGAs have a similar domain organization as mammalian AGAs. They share heterotoma. PLoS ONE 12(7): e0181940. https:// with them key residues for autocatalysis and activity, and the mature - and -subunits also doi.org/10.1371/journal.pone.0181940 α β form an (αβ)2 structure in solution. Interestingly, only one of these AGAs subunits (α for Editor: Erjun Ling, Institute of Plant Physiology and AtAGA and for LhAGA) is glycosylated instead of the two subunits for lysosomal human Ecology Shanghai Institutes for Biological β Sciences, CHINA AGA (hAGA), and these glycosylations are partially resistant to PGNase F treatment. -

Biosynthesis of Natural Products Containing Β-Amino Acids
Natural Product Reports Biosynthesis of natural products containing β -amino acids Journal: Natural Product Reports Manuscript ID: NP-REV-01-2014-000007.R1 Article Type: Review Article Date Submitted by the Author: 21-Apr-2014 Complete List of Authors: Kudo, Fumitaka; Tokyo Institute Of Technology, Department of Chemistry Miyanaga, Akimasa; Tokyo Institute Of Technology, Department of Chemistry Eguchi, T; Tokyo Institute Of Technology, Department of Chemistry and Materials Science Page 1 of 20 Natural Product Reports NPR RSC Publishing REVIEW Biosynthesis of natural products containing βββ- amino acids Cite this: DOI: 10.1039/x0xx00000x Fumitaka Kudo, a Akimasa Miyanaga, a and Tadashi Eguchi *b Received 00th January 2014, We focus here on β-amino acids as components of complex natural products because the presence of β-amino acids Accepted 00th January 2014 produces structural diversity in natural products and provides characteristic architectures beyond that of ordinary DOI: 10.1039/x0xx00000x α-L-amino acids, thus generating significant and unique biological functions in nature. In this review, we first survey the known bioactive β-amino acid-containing natural products including nonribosomal peptides, www.rsc.org/ macrolactam polyketides, and nucleoside-β-amino acid hybrids. Next, the biosynthetic enzymes that form β-amino acids from α-amino acids and de novo synthesis of β-amino acids are summarized. Then, the mechanisms of β- amino acid incorporation into natural products are reviewed. Because it is anticipated that the rational swapping of the β-amino acid moieties with various side chains and stereochemistries by biosynthetic engineering should lead to the creation of novel architectures and bioactive compounds, the accumulation of knowledge regarding β- amino acid-containing natural product biosynthetic machinery could have a significant impact in this field. -

Active Site Tyrosine Is Essential for Amidohydrolase but Not for Esterase
Active site tyrosine is essential for amidohydrolase but not for esterase activity of a class 2 histone deacetylase-like bacterial enzyme Kristin Moreth, Daniel Riester, Christian Hildmann, René Hempel, Dennis Wegener, Andreas Schober, Andreas Schwienhorst To cite this version: Kristin Moreth, Daniel Riester, Christian Hildmann, René Hempel, Dennis Wegener, et al.. Ac- tive site tyrosine is essential for amidohydrolase but not for esterase activity of a class 2 histone deacetylase-like bacterial enzyme. Biochemical Journal, Portland Press, 2006, 401 (3), pp.659-665. 10.1042/BJ20061239. hal-00478649 HAL Id: hal-00478649 https://hal.archives-ouvertes.fr/hal-00478649 Submitted on 30 Apr 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Biochemical Journal Immediate Publication. Published on 12 Oct 2006 as manuscript BJ20061239 Active site tyrosine is essential for amidohydrolase but not for esterase activity of a class 2 histone deacetylase-like bacterial enzyme Kristin Moreth¶, Daniel Riester¶, Christian Hildmann¶, René Hempel¶, Dennis Wegener¶,§,‡, -

Characterization of Aspartylglucosaminidase Activation and Aspartylglucosaminuria Mutations
Arto Pennanen Publications of the National Public Health Institute A 1 / 2004 — INDOOR AIR POLLUTION AND HEALTH RISKS IN FINNISH ICE ARENAS RISKSINFINNISHICE AND HEALTH AIR POLLUTION INDOOR Jani Saarela CHARACTERIZATION OF ASPARTYLGLUCOSAMINIDASE ACTIVATION AND ASPARTYLGLUCOSAMINURIA MUTATIONS ISBN 951-740-485-9 ISSN 0359-3584 ISBN 951-740-486-7 (pdf) Department of Molecular Medicine, ISSN 1458-6290 (pdf) National Public Health Institute, Helsinki, Finland and http://www.ktl.fi /portal/suomi/julkaisut/julkaisusarjat/ Department of Medical Genetics, kansanterveyslaitoksen_julkaisuja_a/ University of Helsinki, Finland Kopijyvä Kuopio 2005 Helsinki 2004 PPennanen_kansi.inddennanen_kansi.indd 1 117.2.20057.2.2005 115:26:195:26:19 CHARACTERIZATION OF ASPARTYLGLUCOSAMINIDASE ACTIVATION AND ASPARTYLGLUCOSAMINURIA MUTATIONS Jani Saarela Department of Molecular Medicine, National Public Health Institute, Helsinki, Finland and Department of Medical Genetics, University of Helsinki, Finland Academic Dissertation To be publicly discussed with the permission of the Medical Faculty of the University of Helsinki, in the lecture room 3 of Biomedicum Helsinki, Haartmaninkatu 8, Helsinki, on January 30th, 2004, at 12 o’clock noon. Helsinki 2004 Supervised by Professor Leena Peltonen-Palotie National Public Health Institute and Department of Medical Genetics University of Helsinki, Helsinki, Finland Reviewed by Professor Ole Kristian Tollersrud and Docent Marc Baumann Department of Medical Biochemistry Protein Chemistry/Proteomics Unit University of Tromsoe and Neuroscience Research Program Tromsoe, Norway University of Helsinki, Helsinki, Finland To be publicly discussed with Professor Marja Makarow Institute of Biotechnology and Department of Applied Biochemistry and Molecular Biology University of Helsinki, Helsinki, Finland Julkaisija-Utgivare-Publisher Kansanterveyslaitos (KTL) Mannerheimintie 166 00300 Helsinki puh. vaihde 09-47441, felefax 09-4744 8408 Folkhälsoinstitutet Mannerheimvägen 166 00300, Helsinki tel. -

United States Patent (10) Patent No.: US 9,562,241 B2 Burk Et Al
USOO9562241 B2 (12) United States Patent (10) Patent No.: US 9,562,241 B2 Burk et al. (45) Date of Patent: Feb. 7, 2017 (54) SEMI-SYNTHETIC TEREPHTHALIC ACID 5,487.987 A * 1/1996 Frost .................... C12N 9,0069 VLAMCROORGANISMIS THAT PRODUCE 5,504.004 A 4/1996 Guettler et all 435,142 MUCONCACID 5,521,075- W I A 5/1996 Guettler et al.a (71) Applicant: GENOMATICA, INC., San Diego, CA 3. A '95 seal. (US) 5,686,276 A 11/1997 Lafend et al. 5,700.934 A 12/1997 Wolters et al. (72) Inventors: Mark J. Burk, San Diego, CA (US); (Continued) Robin E. Osterhout, San Diego, CA (US); Jun Sun, San Diego, CA (US) FOREIGN PATENT DOCUMENTS (73) Assignee: Genomatica, Inc., San Diego, CA (US) CN 1 358 841 T 2002 EP O 494 O78 7, 1992 (*) Notice: Subject to any disclaimer, the term of this (Continued) patent is extended or adjusted under 35 U.S.C. 154(b) by 0 days. OTHER PUBLICATIONS (21) Appl. No.: 14/308,292 Abadjieva et al., “The Yeast ARG7 Gene Product is Autoproteolyzed to Two Subunit Peptides, Yielding Active (22) Filed: Jun. 18, 2014 Ornithine Acetyltransferase,” J. Biol. Chem. 275(15): 11361-11367 2000). (65) Prior Publication Data . al., “Discovery of amide (peptide) bond synthetic activity in US 2014/0302573 A1 Oct. 9, 2014 Acyl-CoA synthetase,” J. Biol. Chem. 28.3(17): 11312-11321 (2008). Aberhart and Hsu, "Stereospecific hydrogen loss in the conversion Related U.S. Application Data of H, isobutyrate to f—hydroxyisobutyrate in Pseudomonas (63) Continuation of application No. -

Eira Kelo | Kelo | 156 | Eira
View metadata, citation and similar papers at core.ac.uk brought to you by CORE dissertations provided by UEF Electronic Publications | 156 | Eira| 156 Kelo | Eira Kelo Catalytic and Therapeutic Characteristics of Human Aspartylglycosaminuria (AGU), Recombinant Glycosylasparaginase an inherited lysosomal storage Catalytic and Therapeutic Characteristics Human of Recombinant Glycosylasparaginase and Bacterial L-asparaginases Eira Kelo disease, is caused by the deficient and Bacterial L-asparaginases activity of a lysosomal enzyme glycosylasparaginase (GA). Catalytic and Therapeutic Loss of GA activity results in the Characteristics of Human accumulation of glycoasparagines in tissues leading to progressive Recombinant Glycosylasparaginase psychomotor retardation and a shortened life span. Currently there is no cure for and Bacterial L-asparaginases AGU. In this study, the effectiveness of enzyme replacement therapy (ERT) with human recombinant GA was evaluated in the mouse model of AGU. Furthermore, the enzymatic properties of GA and bacterial asparaginases were compared. This study reveals new therapeutic and catalytic properties of human GA and bacterial asparaginases. Publications of the University of Eastern Finland Dissertations in Health Sciences Publications of the University of Eastern Finland Dissertations in Health Sciences isbn 978-952-61-1045-5 EIRA KELO EIRA KELO Catalytic and therapeutic characteristics of Catalytic and therapeutic characteristics of human recombinant glycosylasparaginase human recombinant glycosylasparaginase -

WO 2013/184908 A2 12 December 2013 (12.12.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2013/184908 A2 12 December 2013 (12.12.2013) P O P C T (51) International Patent Classification: Jr.; One Procter & Gamble Plaza, Cincinnati, Ohio 45202 G06F 19/00 (201 1.01) (US). HOWARD, Brian, Wilson; One Procter & Gamble Plaza, Cincinnati, Ohio 45202 (US). (21) International Application Number: PCT/US20 13/044497 (74) Agents: GUFFEY, Timothy, B. et al; c/o The Procter & Gamble Company, Global Patent Services, 299 East 6th (22) Date: International Filing Street, Sycamore Building, 4th Floor, Cincinnati, Ohio 6 June 2013 (06.06.2013) 45202 (US). (25) Filing Language: English (81) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (30) Priority Data: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 61/656,218 6 June 2012 (06.06.2012) US DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (71) Applicant: THE PROCTER & GAMBLE COMPANY HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KN, KP, KR, [US/US]; One Procter & Gamble Plaza, Cincinnati, Ohio KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 45202 (US). MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, (72) Inventors: XU, Jun; One Procter & Gamble Plaza, Cincin SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, nati, Ohio 45202 (US).