Pteris Cretica Var
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Presencia Y Distribución De Micofdlas En Gramíneas De Argentina
PRESENCIA Y DISTRIBUCIÓN DE MICOFDLAS EN GRAMÍNEAS DE ARGENTINA por MÓNICA A. LUGO*, ANA M. ANTÓN* & DANIEL CABRAL** Resumen LUGO, M.A., A.M. ANTÓN & D. CABRAL (1998). Presencia y distribución de micofilas en gra míneas de Argentina. Anales Jard. Bot. Madrid 56(1): 15-22. Se realiza un estudio de las micofilas encontradas en gramíneas (Poaceae) de pastizales natu rales de Argentina. La búsqueda del simbionte fúngico se efectuó en plantas vivas y herbori zadas. Se dan a conocer como nuevos hospedantes a Festucafiebrigii Pilg., F. hieronymi Hack, var. expansa (St. Yves) Türpe, F. parodii St. Yves, Melica macra Nees, M. stuckertii Hack., Poa hieronymi Hack., P. holciformis J. Presl, P. lilloi Hack., P. plicata Hack, y P. stuckertii (Hack.) Parodi, y se amplía el área de distribución de la asociación para Festuca arundinacea Schreb., F. hieronymi Hack. var. hieronymi y F. tucumanica E.B. Alexeev. El tipo de interac ción observada corresponde a una simbiosis mutualista. En la mayoría de las especies analiza das el endófito colonizó el parenquima de las cañas y los frutos, salvo en Melica macra. Poa hieronymi y P. plicata, en las que solo se observó en el parenquima. Por otro lado, la presen cia de micofilas presentó variaciones con relación a la especie del hospedante, altitud y locali dad de procedencia. Palabras clave: Endófito fúngico, gramíneas, micofilas, pastizales naturales, Argentina. Abstract LUGO, M.A., A.M. ANTÓN & D. CABRAL (1998). Presence and distribution of mycophyllas in Argentinian grasses. Anales Jard. Bot. Madrid 56(1): 15-22 (in Spanish). The mycophyllas were studied in grasses (Poaceae) from natural Argentinian grasslands. -

Biodiversity Plan for the South East of South Australia 1999
SUMMARY Biodiversity Plan for the South East of South Australia 1999 rks & W Pa i Department for Environment ld l a l i f n e o i t Heritage and Aboriginal Affairs a N South Government of South Australia Australia AUTHORS Tim Croft (National Parks & Wildlife SA) Georgina House (QED) Alison Oppermann (National Parks & Wildlife SA) Ann Shaw Rungie (QED) Tatia Zubrinich (PPK Environment & Infrastructure Pty Ltd) CARTOGRAPHY AND DESIGN National Parks & Wildlife SA (Cover) Geographic Analysis and Research Unit, Planning SA Pierris Kahrimanis PPK Environment & Infrastructure Pty Ltd ACKNOWLEDGEMENTS The authors are grateful to Professor Hugh Possingham, the Nature Conservation Society, and the South Australian Farmers Federation in providing the stimulus for the Biodiversity Planning Program and for their ongoing support and involvement Dr Bob Inns and Professor Possingham have also contributed significantly towards the information and design of the South East Biodiversity Plan. We also thank members of the South East community who have provided direction and input into the plan through consultation and participation in workshops © Department for Environment, Heritage and Aboriginal Affairs, 1999 ISBN 0 7308 5863 4 Cover Photographs (top to bottom) Lowan phebalium (Phebalium lowanense) Photo: D.N. Kraehenbuehl Swamp Skink (Egernia coventryi) Photo: J. van Weenen Jaffray Swamp Photo: G. Carpenter Little Pygmy Possum (Cercartetus lepidus) Photo: P. Aitken Red-necked Wallaby (Macropus rufogriseus) Photo: P. Canty 2 diversity Plan for the South East of South Australia — Summary Foreword The conservation of our natural biodiversity is essential for the functioning of natural systems. Aside from the intrinsic importance of conserving the diversity of species many of South Australia's economic activities are based on the sustainable use, conservation and management of biodiversity. -

Morfología Y Distribución Del Complejo Pteris Cretica L
MEP Candollea 66(1) COMPLET_Mise en page 1 26.07.11 11:03 Page159 Morfología y distribución del complejo Pteris cretica L. (Pteridaceae) para el continente americano Olga Gladys Martínez Abstract Résumé MARTÍNEZ, O. G. (2011). Morphology and distribution of the complex MARTÍNEZ, O. G. (2011). Morphologie et distribution du complexe Pteris Pteris cretica L. (Pteridaceace) for the American continent. Candollea 66: cretica L. (Pteridaceace) pour le continent américain. Candollea 66: 159-180. 159-180. In Spanish, English and French abstracts. En espagnol, résumés anglais et français. The Pteris cretica L. (Pteridaceae) taxonomical complex is Le complexe taxonomique Pteris cretica L. (Pteridaceae) revised for the American continent. It is composed by seven est présenté pour le continent américain. Cette entité est species: Pteris ciliaris D. C. Eaton, Pteris cretica L., Pteris constituée de sept espèces: Pteris ciliaris D. C. Eaton, denticulata Sw., Pteris ensiformis Burm. f., Pteris multifida Pteris cretica L., Pteris denticulata Sw., Pteris ensiformis Poir., Pteris mutilata L. and Pteris tristicula Raddi. Morpho- Burm. f., Pteris multifida Poir., Pteris mutilata L. et Pteris logical characters have been identified in order to distinguish tristicula Raddi. Des caractères morphologiques ont été défi- the members of the group. An identification key is proposed nis afin de distinguer les différents membres de ce complexe. and a diagnostic description, distribution and illustrations are Une clé d’identification est proposée, et pour chaque espèce provided for each species. une description, une carte de distribution et des illustrations sont inclues. Key-words PTERIDACEAE – Pteris – Taxonomy – Morphology – America Dirección del autor: IBIGEO. Herbario MCNS. Facultad de Ciencias Naturales. -

Pteris ×Caridadiae (Pteridaceae), a New Hybrid Fern from Costa Rica
Pteris ×caridadiae (Pteridaceae), a new hybrid fern from Costa Rica 1 2 3 2 WESTON L. TESTO ,JAMES E. WATKINS ,JARMILA PITTERMANN , AND REHMAN MOMIN 1 Pringle Herbarium, Plant Biology Department, University of Vermont, 27 Colchester Avenue, Burlington, VT 05405, USA; e-mail: [email protected] 2 Biology Department, Colgate University, 13 Oak Drive, Hamilton, NY 13346, USA; e-mail: [email protected] 3 Department of Ecology and Evolutionary Biology, University of California Santa Cruz, 1156 High Street, Santa Cruz, CA 95064, USA; e-mail: [email protected] Abstract. Pteris ×caridadiae, a new hybrid fern from Costa Rica, is described and its relationships to its parents and other Pteris species are discussed. This is the first hybrid reported among a taxonomically complicated group of large, tripartite-leaved neotropical Pteris species. Key Words: Fern, hybrid, Pteridaceae, Pteris, systematics, taxonomy. The cosmopolitan fern genus Pteris L. com- upper montane forest adjacent to a small stream. prises approximately 250 species and is most The forest understory at the site was dominated by diverse in low- to mid-elevation forests in the large terrestrial fern taxa, including Diplazium tropics (Chao et al., 2014; Zhang et al., 2014). diplazioides (Klotzsch & H. Karst.) Alston, The group has received limited attention from Dicksonia sellowiana Hook., Thelypteris thomsonii taxonomists, and despite the contributions of re- (Jenman) Proctor, Pteris livida Mett. (Testo 633, cent phylogenetic studies (e.g., Bouma et al., VT), and Pteris podophylla Sw. (Testo 634, MO, 2010;Chaoetal.,2012; Jaruwattanaphan et al., VT). The two Pteris species were particularly abun- 2013; Chao et al., 2014; Zhang et al., 2014), the dant at the site, with numerous large (to 2 m tall) delineation of many species complexes remains sporophytes and sizeable populations of gameto- problematic. -

In Vitro Spore Germination and Gametophytic Growth Development of a Critically Endangered Fern Pteris Tripartita Sw
Vol. 13(23), pp. 2350-2358, 4 June, 2014 DOI: 10.5897/AJB2013.13419 Article Number: 6C227C945161 ISSN 1684-5315 African Journal of Biotechnology Copyright © 2014 Author(s) retain the copyright of this article http://www.academicjournals.org/AJB Full Length Research Paper In vitro spore germination and gametophytic growth development of a critically endangered fern Pteris tripartita Sw. Baskaran Xavier Ravi*, Jeyachandran Robert and Melghias Gabriel Department of Botany, St. Joseph’s College, Tiruchirappalli, Tamil Nadu-620 002, India. Received 24 October, 2013; Accepted 31 March, 2014 The effects of sucrose, pH and plant growth hormones on spore germination percentage and gametophyte growths of Pteris tripartita were studied. Various morphological structures of gametophytes were observed namely, filamentous, spatulate and heart stages in the MS culture medium with hormones. After 15 days, the spores of P. tripartita were sprouted in MS basal medium fortified with pH, sucrose and hormones. Maximum spore germination rates (84%) were observed in 70 g/L of sucrose and 79.33% in pH 5.7. On the other hand, the maximum gametophyte sizes were observed both in 40 and 50 g/l of sucrose on half strength MS medium. The maximum growth of gametophyte lengths (484.39 and 507.72 µm) and widths (846.58 and 1270.98 µm) were observed in both pH 5.7 and 6.7. Among three different hormones, the utmost number or percentage of spores were sprouted in GA3. However, the in vitro cultures of spore having the capability to increase the spore germinated due to addition of adequate nutrition in the culture medium and also reduce the contamination as well as environmental factors. -

A Landscape-Based Assessment of Climate Change Vulnerability for All Native Hawaiian Plants
Technical Report HCSU-044 A LANDscape-bASED ASSESSMENT OF CLIMatE CHANGE VULNEraBILITY FOR ALL NatIVE HAWAIIAN PLANts Lucas Fortini1,2, Jonathan Price3, James Jacobi2, Adam Vorsino4, Jeff Burgett1,4, Kevin Brinck5, Fred Amidon4, Steve Miller4, Sam `Ohukani`ohi`a Gon III6, Gregory Koob7, and Eben Paxton2 1 Pacific Islands Climate Change Cooperative, Honolulu, HI 96813 2 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Hawaii National Park, HI 96718 3 Department of Geography & Environmental Studies, University of Hawai‘i at Hilo, Hilo, HI 96720 4 U.S. Fish & Wildlife Service —Ecological Services, Division of Climate Change and Strategic Habitat Management, Honolulu, HI 96850 5 Hawai‘i Cooperative Studies Unit, Pacific Island Ecosystems Research Center, Hawai‘i National Park, HI 96718 6 The Nature Conservancy, Hawai‘i Chapter, Honolulu, HI 96817 7 USDA Natural Resources Conservation Service, Hawaii/Pacific Islands Area State Office, Honolulu, HI 96850 Hawai‘i Cooperative Studies Unit University of Hawai‘i at Hilo 200 W. Kawili St. Hilo, HI 96720 (808) 933-0706 November 2013 This product was prepared under Cooperative Agreement CAG09AC00070 for the Pacific Island Ecosystems Research Center of the U.S. Geological Survey. Technical Report HCSU-044 A LANDSCAPE-BASED ASSESSMENT OF CLIMATE CHANGE VULNERABILITY FOR ALL NATIVE HAWAIIAN PLANTS LUCAS FORTINI1,2, JONATHAN PRICE3, JAMES JACOBI2, ADAM VORSINO4, JEFF BURGETT1,4, KEVIN BRINCK5, FRED AMIDON4, STEVE MILLER4, SAM ʽOHUKANIʽOHIʽA GON III 6, GREGORY KOOB7, AND EBEN PAXTON2 1 Pacific Islands Climate Change Cooperative, Honolulu, HI 96813 2 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Hawaiʽi National Park, HI 96718 3 Department of Geography & Environmental Studies, University of Hawaiʽi at Hilo, Hilo, HI 96720 4 U. -

Gametophyte Morphology and Development of Six Species of Pteris (Pteridaceae) from Java Island Indonesia
THE JOURNAL OF TROPICAL LIFE SCIENCE OPEN ACCESS Freely available online VOL. 5, NO. 2, pp. 98-104, May, 2015 Gametophyte Morphology and Development of Six Species of Pteris (Pteridaceae) from Java Island Indonesia Dwi Sunarti Puspitasari1, Tatik Chikmawati2*, Titien Ngatinem Praptosuwiryo3 1Plant Biology Graduate Program, Department of Biology, Faculty of Mathematics and Natural Sciences, Bogor Agricultural University, Darmaga Campus, Bogor, Indonesia 2Department of Biology, Faculty of Mathematics and Natural Sciences Bogor Agricultural University, Darmaga Campus, Bogor, Indonesia 3Center for Plant Conservation- Bogor Botanical Gardens, Indonesian Institute of Sciences, Bogor, West Java, Indonesia ABSTRACT The morphology of sporophyte, the type of reproduction, and cytology of Pteris had been reported, while the gametophyte morphology of Pteris in Java island has not been studied yet. The objective of this study was to describe the gametophyte morphology and development of P. biaurita, P. ensiformis, P. exelsa, P. longipinnula, P. tripartita, and P. vittata in Java island. Spores were obtained from fertile leaves of Pteris plants originated from several locations in Java island. The number of spores per sporangium was counted from fresh fertile leaves with mature sporangia. As much as 0.002 g spores was sown in a transparent box with sterile medium contain of ver- miculite, sphagnum moss, and perlite with ratio 2:2:1. The gametophyte development of each species was observed under a microscope every 7 days. The spores of P. ensiformis were germinated faster, ten days after sowing, while the spores of P. longipinnula were germinated slower, 18 days after sowing. The pattern of spore germination is Vittaria-type. -
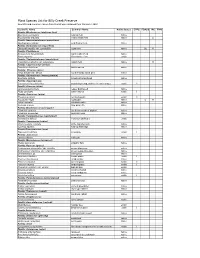
Plant Species List for Billy Creek Preserve Scientific and Common Names from This List Were Obtained from Wunderlin 2003
Plant Species List for Billy Creek Preserve Scientific and Common names from this list were obtained from Wunderlin 2003 Scientific Name Common Name Native Status EPPC FDACS IRC FNAI Family: Blechnaceae (midsorus fern) Blechnum serrulatum swamp fern native Woodwardia areolata netted chain fern native CI Family: Nephrolepidaceae (sword fern) Nephrolepis exaltata wild Boston fern native Family: Osmundaceae (royal fern) Osmunda regalis var. spectabilis royal fern native CE R Family: Pteridaceae Acrostichum danaeifolium giant leather fern native Pteris tripartita Giant brake fern exotic Family: Thelypteridaceae (marsh fern) Thelypteris palustris var. pubescens marsh fern native R Family: Cupressaceae (cedar) Taxodium distichum bald-cypress native Family: Pinaceae (pine) Pinus elliottii var. densa south Florida slash pine native Family: Alismataceae (water plantain) Sagittaria latifolia broadleaf arrowhead native Family: Asparagaceae Sansevieria hyacinthoides bowstring hemp, mother-in-law's tongue exotic II Family: Araceae (arum) Lemna aequinoctialis lesser duckweed native Pistia stratiotes water lettuce exotic I Family: Arecaceae (palm) Phoenix reclinata reclinata palm exotic II Roystonea regia royal palm native E R Sabal palmetto cabbage palm native Serenoa repens saw palmetto native Family: Bromeliaceae (pineapple) Tillandsia setacea southern needleaf airplant native Tillandsia usneoides spanish moss native Family: Commelinaceae (spiderwort) Commelina diffusa common dayflower exotic Family: Cyperaceae (sedge) Rhynchospora colorata white -

Pteris Cretica
Pteris cretica COMMON NAME Cretan brake FAMILY Pteridaceae AUTHORITY Pteris cretica L. FLORA CATEGORY Vascular – Exotic STRUCTURAL CLASS Ferns DISTRIBUTION Naturalised. New Zealand: North and South Islands (widespread from Whangarei south to Banks Peninsula). Indigenous to to the warm- temperate and tropical parts of the Old World. Pteris cretica. Photographer: John Smith- Dodsworth HABITAT Coastal to montane (mostly coastal to lowland). A common weedy fern in many urban parts of New Zealand but also common in less modified areas growing in dense forest, along river, stream and gully banks, on track and roadside cuttings. It can be very common in wasteland areas within cities and towns, and often appears on retaining walls, and even under houses (provided there is some light). FEATURES Large terrestrial ferns. Rhizome short-creeping; scales minute, dark brown. Fronds dimorphic, clustered. Stipes 0.25-0.9 m long, yellow- brown, glabrous. Lamina 0.2-0.6 × 0.1-0.4 m, dark green (occasionally Pteris cretica. Photographer: John Smith- Dodsworth variegated) broadly oblong to oblong, 1-pinnate, often incompletely 2- pinnate (forked) at the base; primary pinnae in 2-7 widely spaced pairs, somewhat ascending, narrowly lanceolate, linear to linear-falcate, tapering to apices and long-acuminate with smooth or minutely denticulate margins, chartaceous, glabrous; rachis not winged or slightly winged at apex. Lower pinnae short-stalked, in mature plants with 1-3 posterior short-stalked free conform pinnules. Upper pinnae sessile, uppermost adnate to rachis. Terminal pinna slightly contracted; apex of sterile pinna, sharply dentate. Veins free, simply or once-forked; false veins absent. Sori continuous; indusium subentire; paraphyses numerous. -

The Island Rule and Its Application to Multiple Plant Traits
The island rule and its application to multiple plant traits Annemieke Lona Hedi Hendriks A thesis submitted to the Victoria University of Wellington in partial fulfilment of the requirements for the degree of Master of Science in Ecology and Biodiversity Victoria University of Wellington, New Zealand 2019 ii “The larger the island of knowledge, the longer the shoreline of wonder” Ralph W. Sockman. iii iv General Abstract Aim The Island Rule refers to a continuum of body size changes where large mainland species evolve to become smaller and small species evolve to become larger on islands. Previous work focuses almost solely on animals, with virtually no previous tests of its predictions on plants. I tested for (1) reduced floral size diversity on islands, a logical corollary of the island rule and (2) evidence of the Island Rule in plant stature, leaf size and petiole length. Location Small islands surrounding New Zealand; Antipodes, Auckland, Bounty, Campbell, Chatham, Kermadec, Lord Howe, Macquarie, Norfolk, Snares, Stewart and the Three Kings. Methods I compared the morphology of 65 island endemics and their closest ‘mainland’ relative. Species pairs were identified. Differences between archipelagos located at various latitudes were also assessed. Results Floral sizes were reduced on islands relative to the ‘mainland’, consistent with predictions of the Island Rule. Plant stature, leaf size and petiole length conformed to the Island Rule, with smaller plants increasing in size, and larger plants decreasing in size. Main conclusions Results indicate that the conceptual umbrella of the Island Rule can be expanded to plants, accelerating understanding of how plant traits evolve on isolated islands. -

Arsenic Tolerance, Accumulation and Elemental Distribution in Twelve Ferns: a Screening Study
AUSTRALASIAN JOURNAL OF ECOTOXICOLOGY Vol. 11, pp. 101-110, 2005 Arsenic tolerance and accumulation in ferns Sridokchan et al ARSENIC TOLERANCE, ACCUMULATION AND ELEMENTAL DISTRIBUTION IN TWELVE FERNS: A SCREENING STUDY Weeraphan Sridokchan1, Scott Markich2 and Pornsawan Visoottiviseth1* 1Department of Biology, Mahidol University, Bangkok 10400, Thailand. 2Aquatic Solutions International, PO Box 3125 Telopea, NSW 2117, Australia. Manuscript received, 15/11/2004; resubmitted, 22/12/2004; accepted, 24/12/2005. ABSTRACT Twelve species of ferns were screened for their ability to tolerate and hyperaccumulate arsenic (As). Ferns were exposed to 50 or 100 mg As L-1 for 7 and 14 days using hydroponic (soil free) experiments. The fronds and roots were analysed for As, selected macronutrients (K, Ca, Mg, P and S) and micronutrients (Al, Fe, Cu and Zn). Five fern species (Asplenium aethiopicum, Asplenium australasicum, Asplenium bulbiferum, Doodia heterophylla and Microlepia strigosa) were found to be sensitive to As. However, only A. australasicum and A. bulbiferum could hyperaccumulate arsenic up to 1240 and 2630 µg As g-1 dry weight (dw), respectively, in their fronds after 7 days at 100 mg As L-1. This is the first known report of ferns that are sensitive to As, yet are As hyperaccumulators. All As tolerant ferns (Adiantum capillus-veneris, Pteris cretica var. albolineata, Pteris cretica var. wimsetti and Pteris umbrosa) were from the Pteridaceae family. P. cretica and P. umbrosa accumulated the majority of As in their fronds (up to 3090 µg As g-1 dw) compared to the roots (up to 760 µg As g-1 dw). In contrast, A. -

Large Trees, Supertrees and the Grass Phylogeny
LARGE TREES, SUPERTREES AND THE GRASS PHYLOGENY Thesis submitted to the University of Dublin, Trinity College for the Degree of Doctor of Philosophy (Ph.D.) by Nicolas Salamin Department of Botany University of Dublin, Trinity College 2002 Research conducted under the supervision of Dr. Trevor R. Hodkinson Department of Botany, University of Dublin, Trinity College Dr. Vincent Savolainen Jodrell Laboratory, Molecular Systematics Section, Royal Botanic Gardens, Kew, London DECLARATION I thereby certify that this thesis has not been submitted as an exercise for a degree at any other University. This thesis contains research based on my own work, except where otherwise stated. I grant full permission to the Library of Trinity College to lend or copy this thesis upon request. SIGNED: ACKNOWLEDGMENTS I wish to thank Trevor Hodkinson and Vincent Savolainen for all the encouragement they gave me during the last three years. They provided very useful advice on scientific papers, presentation lectures and all aspects of the supervision of this thesis. It has been a great experience to work in Ireland, and I am especially grateful to Trevor for the warm welcome and all the help he gave me, at work or outside work, since the beginning of this Ph.D. in the Botany Department. I will always remember his patience and kindness to me at this time. I am also grateful to Vincent for his help and warm welcome during the different periods of time I stayed in London, but especially for all he did for me since my B.Sc. at the University of Lausanne. I wish also to thank Prof.