In Vitro Spore Germination and Gametophytic Growth Development of a Critically Endangered Fern Pteris Tripartita Sw
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gametophyte Morphology and Development of Six Species of Pteris (Pteridaceae) from Java Island Indonesia
THE JOURNAL OF TROPICAL LIFE SCIENCE OPEN ACCESS Freely available online VOL. 5, NO. 2, pp. 98-104, May, 2015 Gametophyte Morphology and Development of Six Species of Pteris (Pteridaceae) from Java Island Indonesia Dwi Sunarti Puspitasari1, Tatik Chikmawati2*, Titien Ngatinem Praptosuwiryo3 1Plant Biology Graduate Program, Department of Biology, Faculty of Mathematics and Natural Sciences, Bogor Agricultural University, Darmaga Campus, Bogor, Indonesia 2Department of Biology, Faculty of Mathematics and Natural Sciences Bogor Agricultural University, Darmaga Campus, Bogor, Indonesia 3Center for Plant Conservation- Bogor Botanical Gardens, Indonesian Institute of Sciences, Bogor, West Java, Indonesia ABSTRACT The morphology of sporophyte, the type of reproduction, and cytology of Pteris had been reported, while the gametophyte morphology of Pteris in Java island has not been studied yet. The objective of this study was to describe the gametophyte morphology and development of P. biaurita, P. ensiformis, P. exelsa, P. longipinnula, P. tripartita, and P. vittata in Java island. Spores were obtained from fertile leaves of Pteris plants originated from several locations in Java island. The number of spores per sporangium was counted from fresh fertile leaves with mature sporangia. As much as 0.002 g spores was sown in a transparent box with sterile medium contain of ver- miculite, sphagnum moss, and perlite with ratio 2:2:1. The gametophyte development of each species was observed under a microscope every 7 days. The spores of P. ensiformis were germinated faster, ten days after sowing, while the spores of P. longipinnula were germinated slower, 18 days after sowing. The pattern of spore germination is Vittaria-type. -

Two Species of Armored Scale Insects (Hemiptera: Diaspididae) Associated with Sori of Ferns Marcelo Guerra Santos¹ & Vera Regina Dos Santos Wolff²
doi:10.12741/ebrasilis.v8i3.492 e-ISSN 1983-0572 Publicação do Projeto Entomologistas do Brasil www.ebras.bio.br Distribuído através da Creative Commons Licence v4.0 (BY-NC-ND) Copyright © EntomoBrasilis Copyright © do(s) Autor(es) Two Species of Armored Scale Insects (Hemiptera: Diaspididae) Associated with Sori of Ferns Marcelo Guerra Santos¹ & Vera Regina dos Santos Wolff² 1. Universidade do Estado do Rio de Janeiro, e-mail: [email protected] (Autor para correspondência). 2. Fundação Estadual de Pesquisa Agropecuária – FEPAGRO, Rio Grande do Sul, e-mail: [email protected]. _____________________________________ EntomoBrasilis 8 (3): 232-234 (2015) Abstract. This note reports the presence of two scale insects species Hemiberlesia palmae (Cockerell) and Pinnaspis strachani (Cooley) (Coccoidea, Diaspididae), associated respectively with Asplenium serratum L. (Aspleniaceae) and Niphidium crassifolium (L.) Lellinger (Polypodiaceae). It is the first record of a fern species as host plant of H. palmae. In both fern species, the diaspidids were found nearby the sori. Keywords: Aspleniaceae; Fern-insect interactions; Polypodiaceae; Pteridophytes; Scale Insect. Duas Espécies de Cochonilhas (Hemiptera: Diaspididae) Associadas com Soros de Samambaias Resumo. A presente comunicação relata a presença de duas espécies de cochonilhas Hemiberlesia palmae (Cockerell) e Pinnaspis strachani (Cooley) (Coccoidea, Diaspididae), associadas respectivamente com Asplenium serratum L. (Aspleniaceae) e Niphidium crassifolium (L.) Lellinger (Polypodiaceae). É o primeiro registro de uma samambaia como planta hospedeira de H. palmae. Nas duas espécies de samambaias, os diaspidídeos encontravam-se concentrados principalmente ao redor dos soros. Palavras-chave: Aspleniaceae; Cochonilhas; Interações samambaia-inseto; Polypodiaceae; Pteridófitas. _____________________________________ nteractions between ferns and insects are more poorly (2003). -
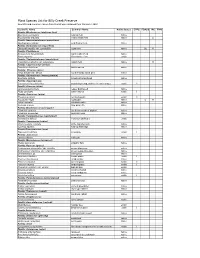
Plant Species List for Billy Creek Preserve Scientific and Common Names from This List Were Obtained from Wunderlin 2003
Plant Species List for Billy Creek Preserve Scientific and Common names from this list were obtained from Wunderlin 2003 Scientific Name Common Name Native Status EPPC FDACS IRC FNAI Family: Blechnaceae (midsorus fern) Blechnum serrulatum swamp fern native Woodwardia areolata netted chain fern native CI Family: Nephrolepidaceae (sword fern) Nephrolepis exaltata wild Boston fern native Family: Osmundaceae (royal fern) Osmunda regalis var. spectabilis royal fern native CE R Family: Pteridaceae Acrostichum danaeifolium giant leather fern native Pteris tripartita Giant brake fern exotic Family: Thelypteridaceae (marsh fern) Thelypteris palustris var. pubescens marsh fern native R Family: Cupressaceae (cedar) Taxodium distichum bald-cypress native Family: Pinaceae (pine) Pinus elliottii var. densa south Florida slash pine native Family: Alismataceae (water plantain) Sagittaria latifolia broadleaf arrowhead native Family: Asparagaceae Sansevieria hyacinthoides bowstring hemp, mother-in-law's tongue exotic II Family: Araceae (arum) Lemna aequinoctialis lesser duckweed native Pistia stratiotes water lettuce exotic I Family: Arecaceae (palm) Phoenix reclinata reclinata palm exotic II Roystonea regia royal palm native E R Sabal palmetto cabbage palm native Serenoa repens saw palmetto native Family: Bromeliaceae (pineapple) Tillandsia setacea southern needleaf airplant native Tillandsia usneoides spanish moss native Family: Commelinaceae (spiderwort) Commelina diffusa common dayflower exotic Family: Cyperaceae (sedge) Rhynchospora colorata white -

The Pteridaceae Family Diversity in Togo
Biodiversity Data Journal 3: e5078 doi: 10.3897/BDJ.3.e5078 Taxonomic Paper The Pteridaceae family diversity in Togo Komla Elikplim Abotsi‡, Aboudou R. Radji‡, Germinal Rouhan§, Jean-Yves Dubuisson§, Kouami Kokou‡ ‡ Université de Lomé, Lomé, Togo § Museum National d'Histoire Naturelle, Paris cedex 05, France Corresponding author: Komla Elikplim Abotsi ([email protected]) Academic editor: Daniele Cicuzza Received: 10 Apr 2015 | Accepted: 10 Jul 2015 | Published: 15 Jul 2015 Citation: Abotsi K, Radji A, Rouhan G, Dubuisson J, Kokou K (2015) The Pteridaceae family diversity in Togo. Biodiversity Data Journal 3: e5078. doi: 10.3897/BDJ.3.e5078 Abstract Background The Pteridaceae family is the largest fern family in Togo by its specific and generic diversity. Like all other families of ferns in the country, Pteridaceae are poorly studied and has no identification key. The objective of this study is to perform a taxonomic revision and list establishment of this family of leptosporangiate ferns in the light of current available knowledge about the family. Pteridaceae was also assessed in terms of its diversity and conservation status, this was conducted through the recent field data and the existing herbaria specimens. The current study permits to confirm the presence of Pteris similis Kuhn. which brought the number of Pteridaceae to 17 in Togo. New information This study provides first local scientific information about the fern flora of Togo. It confirmed the presence of Pteris similis Kuhn. in Togo and brought the Pteridaceae family diversity to 17 species. A species identification key is provided for the easy identification of the Pteridaceae of Togo. -

DENR Administrative Order. 2017. Updated National List of Threatened
Republic of the Philippines Department of Environment and Natural Resources Visayas Avenue, Diliman, Quezon City Tel. Nos. 929-6626; 929-6628; 929-6635;929-4028 929-3618;426-0465;426-0001; 426-0347;426-0480 VOiP Trunkline (632) 988-3367 Website: http://www.denr.gov.ph/ E-mail: [email protected] DENR ADMINISTRATIVE ORDER No. 2017----------11 MAVO 2 2017 SUBJECT UPDATED NATIONAL LIST OF THREATENED PHILIPPINE PLANTS AND THEIR CATEGORIES Pursuant to Section 22 of Republic Act No. 9147otherwise known as the "Wildlife Resources Conservation and Protection Act"and in accordance with Section 6 of DENR Administrative Order No. 2007-01 (Establishing the National List of Threatened Philippines Plants and their Categories and the List of Other Wildlife Species), the National List of Threatened Philippine Plants and their categories, is hereby updated. Section 1. Definition of Terms. As used in this Order, the following terms shall mean as follows: a. CITES - refers to the Convention on International Trade in Endangered Species of Wild Fauna and Flora, a treaty regulating international trade of fauna and flora listed in its Appendices; CITES Appendix I - species threatened with extinction, which are or may be affected by trade. International (commercial) trade in wild-taken specimens is generally prohibited. CITES Appendix II -species not necessarily threatened with extinction, but for which trade must be controlled to avoid their becoming so, and species that resemble species already included in Appendix II. International trade is permitted but regulated through appropriate permits/certificates. CITES Appendix III - species included at the request of a Party that already regulates trade in the species and that needs the cooperation of other countries to prevent unsustainable or illegal exploitation. -

Sporophyte and Gametophyte Development of Platycerium Coronarium (Koenig) Desv
Saudi Journal of Biological Sciences (2010) 17,13–22 King Saud University Saudi Journal of Biological Sciences www.ksu.edu.sa www.sciencedirect.com ORIGINAL ARTICLE Sporophyte and gametophyte development of Platycerium coronarium (Koenig) Desv. and P. grande (Fee) C. Presl. (Polypodiaceae) through in vitro propagation Reyno A. Aspiras Department of Biology, College of Arts and Sciences, Central Mindanao University, University Town, Musuan, Bukidnon, Philippines Available online 22 December 2009 KEYWORDS Abstract The sporophyte and gametophyte development of Platycerium coronarium and P. grande Propagation techniques; were compared through ex situ propagation using in vitro culture technique and under greenhouse Endangered species; and field conditions. Staghorn ferns; The morphology of the sporophyte and gametophyte, type of spore germination and prothallial Sporophyte; development of P. coronarium and P. grande were documented. Gametophytes of P. coronarium Gametophyte and P. grande were cultured in vitro using different media. The gametophytes were then transferred and potted in sterile chopped Cyathea spp. (anonotong) roots and garden soil for sporophyte forma- tion. Sporophytes (plantlets) of the two Platycerium species were attached on the slabs of anonotong and on branches and trunks of Swietenia macrophylla (mahogany) under greenhouse and field condi- tions. Sporophyte morphology of P. coronarium and P. grande varies but not their gametophyte morphol- ogy. P. coronarium and P. grande exhibited rapid spore germination and gametophyte development in both spore culture medium and Knudson C culture medium containing 2% glucose. Gametophytes of P. coronarium and P. grande transferred to potting medium produced more number of sporophytes while the gametophytes inside the culture media did not produce sporophytes. -

P;J/AI1)~!Jp/L
~. p;J/AI1) ~!Jp/l \II ~iJIO.IYO-r.?(}) l .._ A REVIEW AND ASSESSMENT OF THE FLORISTIC KNOWLEDGE OF SAMAR ISLAND Based on literature, PNH Records and Current Knowledge' ..l ..I .., USAID ******* 'I.; , I:,•• A REVIEW AND ASSESSMENT OF THE FLORISTIC KNOWLEDGE OF SAMAR ISLAND Based on literature, PNH Records and Current Knowledge' by DOMINGO A. MADUUD' Specialist for Flora November 30, 2000 Samar Island Biodiversity Study (SAMBIO) Resources, Environment and Economics Center for Studies, Inc. (REECS) In association with Orient Integrated Development Consultants, Inc. (OIDCI) Department of Environment and Natural Resources (DENR) I This publication was made possible through support provided by the U. S. Agency for International Development, under the terms of Grant No. 492-G-OO-OO-OOOOT-OO. The opinions expressed herein are those of the author and do not necessarily reflect the views of the U. S. Agency for International DevelopmenL 2 The author, Dr. Domingo Madulid, is the Floristic Assessment Specialist of SAMBIO, REECS. / TABLE OF CONTENTS List ofTables Executive Summary.................................................................................... iv 1. INTRODUCTION . 1 2. METHODOLOGy . 2 2.1 Brief Historical Account of Botanical Explorations in Samar (based on records of the Philippine National Herbarium) . 2 3. BOTANICAL SIGNIFICANCE OF SAMAR ISLAND.............................. 5 3.1 Rare, Endangered, Endemic, and Useful Plants of Samar................ 5 3.2 Vegetation Types in Samar Island............................................. 7 4. ASSESSMENT OF BOTANICAL INFORMATION AVAILABLE............... 8 4.1 Plant Diversity Assessment Inside the Forest Resource Assessment Transect Lines........................................................................ 9 4.2 List of Threatened Plants Found in the Transect Plots and Adjoining Areas...................................................................... 10 1iIII. 4.3 Species Diversity of Economic Plants from the Transect.............. -

Rare and Threatened Pteridophytes of Asia 2. Endangered Species of India — the Higher IUCN Categories
Bull. Natl. Mus. Nat. Sci., Ser. B, 38(4), pp. 153–181, November 22, 2012 Rare and Threatened Pteridophytes of Asia 2. Endangered Species of India — the Higher IUCN Categories Christopher Roy Fraser-Jenkins Student Guest House, Thamel. P.O. Box no. 5555, Kathmandu, Nepal E-mail: [email protected] (Received 19 July 2012; accepted 26 September 2012) Abstract A revised list of 337 pteridophytes from political India is presented according to the six higher IUCN categories, and following on from the wider list of Chandra et al. (2008). This is nearly one third of the total c. 1100 species of indigenous Pteridophytes present in India. Endemics in the list are noted and carefully revised distributions are given for each species along with their estimated IUCN category. A slightly modified update of the classification by Fraser-Jenkins (2010a) is used. Phanerophlebiopsis balansae (Christ) Fraser-Jenk. et Baishya and Azolla filiculoi- des Lam. subsp. cristata (Kaulf.) Fraser-Jenk., are new combinations. Key words : endangered, India, IUCN categories, pteridophytes. The total number of pteridophyte species pres- gered), VU (Vulnerable) and NT (Near threat- ent in India is c. 1100 and of these 337 taxa are ened), whereas Chandra et al.’s list was a more considered to be threatened or endangered preliminary one which did not set out to follow (nearly one third of the total). It should be the IUCN categories until more information realised that IUCN listing (IUCN, 2010) is became available. The IUCN categories given organised by countries and the global rarity and here apply to political India only. -

Botanical Survey of the War in the Pacific National Historical Park Guam, Mariana Islands
PACIFIC COOPERATIVE STUDIES UNIT UNIVERSITY OF HAWAI`I AT MĀNOA Dr. David C. Duffy, Unit Leader Department of Botany 3190 Maile Way, St. John #408 Honolulu, Hawai’i 96822 Technical Report 161 Botanical survey of the War in the Pacific National Historical Park Guam, Mariana Islands July 2008 Joan M. Yoshioka 1 1 Pacific Cooperative Studies Unit (University of Hawai`i at Mānoa), NPS Inventory and Monitoring Program, Pacific Island Network, PO Box 52, Hawai`i National Park, HI 96718 PCSU is a cooperative program between the University of Hawai`i and U.S. National Park Service, Cooperative Ecological Studies Unit. Organization Contact Information: Inventory and Monitoring Program, Pacific Island Network, PO Box 52, Hawaii National Park, HI 96718, phone: 808-985-6183, fax: 808-985-6111 Recommended Citation: Yoshioka, J. M. 2008. Botanical survey of the War in the Pacific National Historical Park Guam, Mariana Islands. Pacific Cooperative Studies Unit Technical Report 161, University of Hawai`i at Manoa, Department of Botany, Honolulu, HI. Key words: Vegetation types, Vegetation management, Alien species, Endemic species, Checklist, Ferns, Flowering plants Place key words: War in the Pacific National Historical Park, Guam Editor: Clifford W. Morden, PCSU Deputy Director (Mail to: mailto:[email protected]) i Table of Contents List of Tables......................................................................................................iii List of Figures ....................................................................................................iii -

Catergory A. Critically Endangered Species
THE NATIONAL LIST OF THREATENED PHILIPPINE PLANTS AND THEIR CATEGORIES, AND THE LIST OF OTHER WILDLIFE SPECIES Definition of Terms. 1. Common name – refers to the adopted name of a species as is widely used in the country; may be based on English or other foreign name, or Tagalog name, or when no local or vernacular name is available is derived from the meaning of its scientific name; 2. Critically Endangered Species – refers to a species or subspecies facing extremely high risk of extinction in the wild in the immediate future. This shall include varieties, formae or other infraspecific categories; 3. Endangered Species - refers to a species or subspecies that is not critically endangered but whose survival in the wild is unlikely if the causal factors continue operating. This shall include varieties, formae or other infraspecific categories; 4. Other Threatened Species – refers to a species or subspecies that is not critically endangered, endangered nor vulnerable but is under threat from adverse factors, such as over collection, throughout its range and is likely to move to the vulnerable category in the near future. This shall include varieties, formae or other infraspecific categories; 5. Other Wildlife Species – refers to non-threatened species of plants that have the tendency to become threatened due to destruction of habitat or other similar causes as may be listed by the Secretary upon the recommendation of the National Wildlife Management Committee. This shall include varieties, formae or other infraspecific categories; 6. Threatened Species – is a general term to denote species or subspecies that is considered as critically endangered, endangered, vulnerable or other accepted categories of wildlife whose populations are at risk of extinction. -

National Wetland Plant List: 2016 Wetland Ratings
Lichvar, R.W., D.L. Banks, W.N. Kirchner, and N.C. Melvin. 2016. The National Wetland Plant List: 2016 wetland ratings. Phytoneuron 2016-30: 1–17. Published 28 April 2016. ISSN 2153 733X THE NATIONAL WETLAND PLANT LIST: 2016 WETLAND RATINGS ROBERT W. LICHVAR U.S. Army Engineer Research and Development Center Cold Regions Research and Engineering Laboratory 72 Lyme Road Hanover, New Hampshire 03755-1290 DARIN L. BANKS U.S. Environmental Protection Agency, Region 7 Watershed Support, Wetland and Stream Protection Section 11201 Renner Boulevard Lenexa, Kansas 66219 WILLIAM N. KIRCHNER U.S. Fish and Wildlife Service, Region 1 911 NE 11 th Avenue Portland, Oregon 97232 NORMAN C. MELVIN USDA Natural Resources Conservation Service Central National Technology Support Center 501 W. Felix Street, Bldg. 23 Fort Worth, Texas 76115-3404 ABSTRACT The U.S. Army Corps of Engineers (Corps) administers the National Wetland Plant List (NWPL) for the United States (U.S.) and its territories. Responsibility for the NWPL was transferred to the Corps from the U.S. Fish and Wildlife Service (FWS) in 2006. From 2006 to 2012 the Corps led an interagency effort to update the list in conjunction with the U.S. Environmental Protection Agency (EPA), the FWS, and the USDA Natural Resources Conservation Service (NRCS), culminating in the publication of the 2012 NWPL. In 2013 and 2014 geographic ranges and nomenclature were updated. This paper presents the fourth update of the list under Corps administration. During the current update, the indicator status of 1689 species was reviewed. A total of 306 ratings of 186 species were changed during the update. -

Ferns and Lycophytes of Mt. Tago Range, Bukidnon, Southern Philippines: Species Richness, Distribution, and Conservation Status
Philippine Journal of Science 149 (3-a): 773-790, October 2020 ISSN 0031 - 7683 Date Received: 22 May 2020 Ferns and Lycophytes of Mt. Tago Range, Bukidnon, Southern Philippines: Species Richness, Distribution, and Conservation Status Fulgent P. Coritico1,2*, Victor B. Amoroso1,2, Florfe M. Acma1,2, Yvonne Love L. Cariño1, and Peter W. Fritsch3 1Center for Biodiversity Research and Extension in Mindanao 2Department of Biology, College of Arts and Sciences Central Mindanao University (CMU), Musuan, Bukidnon 8710 Philippines 3Botanical Research Institute of Texas (BRIT) 1700 University Drive, Fort Worth, TX 76107-3400 USA The species of ferns and lycophytes of Mt. Tago range are here documented in a checklist, along with information on their distribution and conservation status. The list is based on a comprehensive field survey conducted by the authors in 2018 and 2019 and comprises 203 species belonging to 29 families and 89 genera. Of these species, 187 are ferns and 16 are lycophytes. Eighty-six species are epiphytes, 85 are terrestrial, 12 are tree ferns, 6 are hemiepiphytes, and 14 species have more than one growth form. The number of species constitutes about 19% and 33% of the total number of pteridophyte species in the Philippines and Mindanao Island, respectively. The highest species richness was observed in the upper montane rainforest. Seventeen species are classified as broadly distributed Philippine endemics whereas four species are endemic to Mindanao. One species (Alsophila commutata Mett.) was newly documented for the Philippines and three species [Pteris whitfordii Copel., Selliguea elmeri (Copel.) Ching, and Selliguea pyrolifolia (Goldm.) Hovenkamp] were newly documented for Mindanao Island.