Gametophyte Morphology and Development of Six Species of Pteris (Pteridaceae) from Java Island Indonesia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reproduction in Plants Which But, She Has Never Seen the Seeds We Shall Learn in This Chapter
Reproduction in 12 Plants o produce its kind is a reproduction, new plants are obtained characteristic of all living from seeds. Torganisms. You have already learnt this in Class VI. The production of new individuals from their parents is known as reproduction. But, how do Paheli thought that new plants reproduce? There are different plants always grow from seeds. modes of reproduction in plants which But, she has never seen the seeds we shall learn in this chapter. of sugarcane, potato and rose. She wants to know how these plants 12.1 MODES OF REPRODUCTION reproduce. In Class VI you learnt about different parts of a flowering plant. Try to list the various parts of a plant and write the Asexual reproduction functions of each. Most plants have In asexual reproduction new plants are roots, stems and leaves. These are called obtained without production of seeds. the vegetative parts of a plant. After a certain period of growth, most plants Vegetative propagation bear flowers. You may have seen the It is a type of asexual reproduction in mango trees flowering in spring. It is which new plants are produced from these flowers that give rise to juicy roots, stems, leaves and buds. Since mango fruit we enjoy in summer. We eat reproduction is through the vegetative the fruits and usually discard the seeds. parts of the plant, it is known as Seeds germinate and form new plants. vegetative propagation. So, what is the function of flowers in plants? Flowers perform the function of Activity 12.1 reproduction in plants. Flowers are the Cut a branch of rose or champa with a reproductive parts. -

Pteris Carsei
Pteris carsei COMMON NAME Coastal brake, netted brake SYNONYMS Pteris comans G.Forst. (misapplied name) FAMILY Pteridaceae AUTHORITY Pteris carsei Braggins et Brownsey FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes ENDEMIC GENUS Pteris carsei Motuoruhi, Coromandel. No Photographer: John Smith-Dodsworth ENDEMIC FAMILY No STRUCTURAL CLASS Ferns NVS CODE PTECAR CHROMOSOME NUMBER 2n = 58, 60 Pteris carsei Motuoruhi, Coromandel. Photographer: John Smith-Dodsworth CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened DISTRIBUTION Endemic. New Zealand: Kermadec Islands (Raoul, the Meyers Islands and Macauley Island), Three Kings and North Island from North Cape to Bay of Plenty in the east and Awhitu Peninsula in the west with an outlying population near Mokau. HABITAT Coastal in forest especially on the sides of gullies, on banks and in valley heads. A very common offshore island fern FEATURES Terrestrial ferns. Rhizomes short, erect, scaly. Stipes 0.25-0.6 m long, pale brown, glabrous or scaly at very base. Laminae 0.2-1.8 × 0.15-0.9 m, dark green to yellow-green, 2-3-pinnate at base, ovate, coriaceous, veins reticulate. Pinnae not overlapping; most lower secondary pinnae adnate. Ultimate segments 10-55 × 5-10 mm, oblong, apices tapering or bluntly pointed, margins toothed. Sori continuous along pinna margins on a marginal vein, protected by a membranous inrolled pinna margins. SIMILAR TAXA Pteris carsei is easily distinguished from all other New Zealand Pteris by the coriaceous (leathery) fronds, reticulate venation, overlapping pinnae and large ultimate segments. The only other Pteris with reticulate venation are P. -

Metabolic Adaptations to Arsenic-Induced Oxidative Stress in Pterisvittata L and Pterisensiformis L
Plant Science 170 (2006) 274–282 www.elsevier.com/locate/plantsci Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L and Pteris ensiformis L Nandita Singh a, Lena Q. Ma b,*, Mrittunjai Srivastava b, Bala Rathinasabapathi c a National Botanical Research Institute, Rana Pratap Marg, Lucknow 226001, India b Soil and Water Science Department, University of Florida, Box 110290, Gainesville, FL 32611-0290, USA c Horticultural Sciences Department, University of Florida, Gainesville, FL 32611, USA Received 14 July 2005; received in revised form 12 August 2005; accepted 19 August 2005 Available online 8 September 2005 Abstract This study examined the metabolic adaptations of Pteris vittata L, an arsenic hyperaccumulator, under arsenic stress as compared to Pteris ensiformis, a non-arsenic hyperaccumulator. Both plants were grown hydroponically in 20% Hoagland medium in controlled conditions and were treated with 0, 133 or 267 mM arsenic as sodium arsenate for 1, 5 or 10 d. The fern fronds were analysed for differences in oxidative stress and antioxidant capacities after arsenic exposure. Upon exposure to 133 mM arsenic, concentrations of chlorophyll, protein and carotenoids increased in P. vittata whereas they decreased in P. ensiformis. The H2O2 and TBARs concentrations were greater in P. ensiformis than P. vittata in all treatments, indicating greater production of reactive oxygen species (ROS) by P. ensiformis. The levels of ascorbate and glutathione, and their reduced/oxidized ratios in the fronds of P. vittata of the control was much greater than P. ensiformis indicating that P. vittata has an inherently greater antioxidant potential than P. ensiformis. The lower levels of antioxidant compounds (ascorbate, carotenoids and glutathione) in P. -

Heterospory: the Most Iterative Key Innovation in the Evolutionary History of the Plant Kingdom
Biol. Rej\ (1994). 69, l>p. 345-417 345 Printeii in GrenI Britain HETEROSPORY: THE MOST ITERATIVE KEY INNOVATION IN THE EVOLUTIONARY HISTORY OF THE PLANT KINGDOM BY RICHARD M. BATEMAN' AND WILLIAM A. DiMlCHELE' ' Departments of Earth and Plant Sciences, Oxford University, Parks Road, Oxford OXi 3P/?, U.K. {Present addresses: Royal Botanic Garden Edinburiih, Inverleith Rojv, Edinburgh, EIIT, SLR ; Department of Geology, Royal Museum of Scotland, Chambers Street, Edinburgh EHi ijfF) '" Department of Paleohiology, National Museum of Natural History, Smithsonian Institution, Washington, DC^zo^bo, U.S.A. CONTENTS I. Introduction: the nature of hf^terospon' ......... 345 U. Generalized life history of a homosporous polysporangiophyle: the basis for evolutionary excursions into hetcrospory ............ 348 III, Detection of hcterospory in fossils. .......... 352 (1) The need to extrapolate from sporophyte to gametophyte ..... 352 (2) Spatial criteria and the physiological control of heterospory ..... 351; IV. Iterative evolution of heterospory ........... ^dj V. Inter-cladc comparison of levels of heterospory 374 (1) Zosterophyllopsida 374 (2) Lycopsida 374 (3) Sphenopsida . 377 (4) PtiTopsida 378 (5) f^rogymnospermopsida ............ 380 (6) Gymnospermopsida (including Angiospermales) . 384 (7) Summary: patterns of character acquisition ....... 386 VI. Physiological control of hetcrosporic phenomena ........ 390 VII. How the sporophyte progressively gained control over the gametophyte: a 'just-so' story 391 (1) Introduction: evolutionary antagonism between sporophyte and gametophyte 391 (2) Homosporous systems ............ 394 (3) Heterosporous systems ............ 39(1 (4) Total sporophytic control: seed habit 401 VIII. Summary .... ... 404 IX. .•Acknowledgements 407 X. References 407 I. I.NIRODUCTION: THE NATURE OF HETEROSPORY 'Heterospory' sensu lato has long been one of the most popular re\ie\v topics in organismal botany. -

In Vitro Spore Germination and Gametophytic Growth Development of a Critically Endangered Fern Pteris Tripartita Sw
Vol. 13(23), pp. 2350-2358, 4 June, 2014 DOI: 10.5897/AJB2013.13419 Article Number: 6C227C945161 ISSN 1684-5315 African Journal of Biotechnology Copyright © 2014 Author(s) retain the copyright of this article http://www.academicjournals.org/AJB Full Length Research Paper In vitro spore germination and gametophytic growth development of a critically endangered fern Pteris tripartita Sw. Baskaran Xavier Ravi*, Jeyachandran Robert and Melghias Gabriel Department of Botany, St. Joseph’s College, Tiruchirappalli, Tamil Nadu-620 002, India. Received 24 October, 2013; Accepted 31 March, 2014 The effects of sucrose, pH and plant growth hormones on spore germination percentage and gametophyte growths of Pteris tripartita were studied. Various morphological structures of gametophytes were observed namely, filamentous, spatulate and heart stages in the MS culture medium with hormones. After 15 days, the spores of P. tripartita were sprouted in MS basal medium fortified with pH, sucrose and hormones. Maximum spore germination rates (84%) were observed in 70 g/L of sucrose and 79.33% in pH 5.7. On the other hand, the maximum gametophyte sizes were observed both in 40 and 50 g/l of sucrose on half strength MS medium. The maximum growth of gametophyte lengths (484.39 and 507.72 µm) and widths (846.58 and 1270.98 µm) were observed in both pH 5.7 and 6.7. Among three different hormones, the utmost number or percentage of spores were sprouted in GA3. However, the in vitro cultures of spore having the capability to increase the spore germinated due to addition of adequate nutrition in the culture medium and also reduce the contamination as well as environmental factors. -

Morphological and Anatomical Adaptations to Dry, Shady Environments in Adiantum Reniforme Var
Morphological and anatomical adaptations to dry, shady environments in Adiantum reniforme var. sinense (Pteridaceae) Di Wu1, Linbao Li1, Xiaobo Ma1, Guiyun Huang1 and Chaodong Yang2 1 Rare Plants Research Institute of Yangtze River, Three Gorges Corporation, Yichang, China 2 Engineering Research Center of Ecology and Agriculture Use of Wetland, Ministry of Education, Yangtze University, Jingzhou, China ABSTRACT The natural distribution of the rare perennial fern Adiantum reniforme var. sinense (Pteridaceae), which is endemic to shady cliff environments, is limited to small areas of Wanzhou County, Chongqing, China. In this study, we used brightfield and epifluorescence microscopy to investigate the anatomical structures and histochemical features that may allow this species to thrive in shady, dry cliff environments. The A. reniforme var. sinense sporophyte had a primary structure and a dictyostele. The plants of this species had an endodermis, sclerenchyma layers and hypodermal sterome, reflecting an adaption to dry cliff environments. Blades had a thin cuticle and isolateral mesophyll, suggesting a tolerance of shady environments. These characteristics are similar to many sciophyte ferns such as Lygodium japonicum and Pteris multifida. Thus, the morphological and anatomical characteristics of A. reniforme var. sinense identified in this study are consistent with adaptations to shady, dry cliff environments. Subjects Conservation Biology, Plant Science Keywords Endodermis, Dictyostele, Sclerenchyma layer, Suberin lamellae, Thin cuticle Submitted 14 April 2020 Accepted 24 August 2020 INTRODUCTION Published 30 September 2020 Adiantum reniforme var. sinense (Pteridaceae, subfamily Vittarioideae) is a rare Corresponding authors Guiyun Huang, cliff-dwelling perennial pteridophyte, with a natural distribution limited to small areas of [email protected] Wanzhou County, Chongqing, China. -

Pteris Vittata Arsenic Accumulation Only Partially Explains Soil Arsenic Depletion During Field-Scale Phytoextraction
Article Pteris vittata Arsenic Accumulation Only Partially Explains Soil Arsenic Depletion during Field-Scale Phytoextraction Sarick Matzen 1, Sirine Fakra 2, Peter Nico 3 and Céline Pallud 1,* 1 Department of Environmental Science, Policy, and Management, University of California-Berkeley, 130 Mulford Hall, Berkeley, CA 94720, USA; [email protected] 2 Advanced Light Source, Lawrence Berkeley National Laboratory, One Cyclotron Road, Berkeley, CA 94720, USA; [email protected] 3 Earth and Environmental Sciences Area, Lawrence Berkeley National Laboratory, One Cyclotron Road, Berkeley, CA 94720, USA; [email protected] * Correspondence: [email protected] Received: 12 October 2020; Accepted: 29 November 2020; Published: 4 December 2020 Abstract: Soil arsenic heterogeneity complicates our understanding of phytoextraction rates during arsenic phytoextraction with Pteris vittata, including in response to rate stimulation with nutrient treatments. In a 58-week arsenic phytoextraction field study, we determined the effects of soil arsenic concentrations, fertilizer application, and mycorrhizal fungi inoculation on P. vittata arsenic uptake rates, soil arsenic depletion, and arsenic soil–plant mass balances. Initial soil arsenic concentrations were positively correlated with arsenic uptake rates. Soil inoculation with mycorrhizal fungus Funneliformis mosseae led to 1.5–2 times higher fern aboveground biomass. Across all treatments, ferns accumulated a mean of 3.6% of the initial soil arsenic, and mean soil arsenic concentrations decreased by up to 44%. At depths of 0–10 cm, arsenic accumulation in P. vittata matched soil arsenic depletion. However, at depths of 0–20 cm, fern arsenic accumulation could not account for 61.5% of the soil arsenic depletion, suggesting that the missing arsenic could have been lost to leaching. -

Pteris Vittata L
Pteris vittata L. Origin and diffusion Origin: tropical areas Distribution: sub-cosmopolitan Invasive potential: medium Source: www.plants.usda.gov Photo: G. Nicolella Photos: L. Passatore Introduction P. vittata is a perennial fern, native to tropical regions and naturalized throughout much of the world It has pinnate fronds tufted or closely spaced, herbaceous to slightly coriaceous. It is a pioneer species, typically growing in humid environments like most of the ferns, it colonizes walls, cliffs and rocks, usually in shade. This species has been one of the most investigated plant for phytotechnologies purposes, as it has been found to be able to take up prodigious amounts of arsenic from soil and sequester it mostly in the above-ground biomass. Common names: Chinese brake (English), Pteride a foglie lunghe (Italian) Description Life-form and periodicity: perennial, evergreen Height: 0,3-0,5 m Roots habit: strong creeping rhizome, with very abundant and thin rhizoids (which serve no other function than attachment). Maximum root-system depth: 30 cm. Culm/Stem/Trunk: - Crown: - Fam. Poaceae Description Leaf: tufted fronds, arching, leathery, pinnate, with an elliptic shape. Rate of transpiration: - Reproductive structure: fertile fronds bear sporangia (spore producing structures) on the underside of fronds. A group of sporangia is referred to as a sorus. Sori are disposed in a sub-marginal line along both sides of the pinna, from near the base to near the tip. Propagative structure: spores Development Sexual propagation: The drying of the sporangia catapults the mature spores from the fern in order to disperse spores outside the immediate neighborhood of the parent, thus aiding in wide-range dispersal. -

THE DIVERSITY of EPIPHYTIC FERN on the OIL PALM TREE (Elaeis Guineensis Jacq.) in PEKANBARU, RIAU
JURNAL BIOLOGI XVII (2) : 51 - 55 ISSN : 1410 5292 THE DIVERSITY OF EPIPHYTIC FERN ON THE OIL PALM TREE (Elaeis guineensis Jacq.) IN PEKANBARU, RIAU KEANEKARAGAMAN JENIS PAKU EPIFIT YANG TUMBUH PADA BATANG KELAPA SAWIT (Elaeis guineensis Jacq.) DI PEKANBARU, RIAU NERY SOFIYANTI Department of Biology, Faculty of Mathematic and Resource Sciences, University of Riau. Kampus Bina Widya Simpang Baru, Panam, Pekanbaru, Riau. Email: [email protected] INTISARI Kelapa sawit (Elaeis guineensis) merupakan salah satu komoditas utama di Provinsi Riau. Secara morfologi, batang kelapa sawit mempunyai lingkungan yang sesuai bagi pertumbuhan paku-pakuan epifit, karena bagian pangkal tangkai daun yang melebar sehingga dapat menampung serasah organik dan materi anorganik lainnya. Tujuan dari kajian ini adalah untuk mengetahui keanekaragaman jenis paku epifit yang tumbuh pada batang kelapa sawit. Sebanyak 125 individu kelapa sawit dari tujuh area kajian di Pekanbaru, Riau telah diteliti. Jumlah jenis paku epifit yang diidentifikasi pada penelitian ini adalah 16 jenis yang tergolong enam famili. Kata kunci : paku epifit, kelapa sawit, Pekanbaru ABSTRACT Oil palm (Elaeis guineensis) is one main commodity in Riau Province. Morphologically, the trunk of oil palm has suitable environment to the growth of epiphytic fern, due to its broaden base of petiole that may accumulate organic and inorganic debris. The objective of this study was to investigate the diversity of epiphytic fern on the oil palm tree. A total of 125 oil palm trees from seven study sites in Pekanbaru, Riau were observed. The number of epiphytic ferns identified in this study was 16 species belongs to six families. Keyword: epiphytic fern, oil palm tree, Pekanbaru INTRODUCTION flowers. -
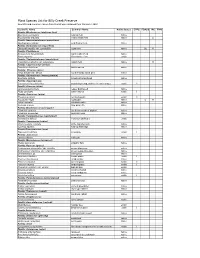
Plant Species List for Billy Creek Preserve Scientific and Common Names from This List Were Obtained from Wunderlin 2003
Plant Species List for Billy Creek Preserve Scientific and Common names from this list were obtained from Wunderlin 2003 Scientific Name Common Name Native Status EPPC FDACS IRC FNAI Family: Blechnaceae (midsorus fern) Blechnum serrulatum swamp fern native Woodwardia areolata netted chain fern native CI Family: Nephrolepidaceae (sword fern) Nephrolepis exaltata wild Boston fern native Family: Osmundaceae (royal fern) Osmunda regalis var. spectabilis royal fern native CE R Family: Pteridaceae Acrostichum danaeifolium giant leather fern native Pteris tripartita Giant brake fern exotic Family: Thelypteridaceae (marsh fern) Thelypteris palustris var. pubescens marsh fern native R Family: Cupressaceae (cedar) Taxodium distichum bald-cypress native Family: Pinaceae (pine) Pinus elliottii var. densa south Florida slash pine native Family: Alismataceae (water plantain) Sagittaria latifolia broadleaf arrowhead native Family: Asparagaceae Sansevieria hyacinthoides bowstring hemp, mother-in-law's tongue exotic II Family: Araceae (arum) Lemna aequinoctialis lesser duckweed native Pistia stratiotes water lettuce exotic I Family: Arecaceae (palm) Phoenix reclinata reclinata palm exotic II Roystonea regia royal palm native E R Sabal palmetto cabbage palm native Serenoa repens saw palmetto native Family: Bromeliaceae (pineapple) Tillandsia setacea southern needleaf airplant native Tillandsia usneoides spanish moss native Family: Commelinaceae (spiderwort) Commelina diffusa common dayflower exotic Family: Cyperaceae (sedge) Rhynchospora colorata white -

Characterization of Antimicrobial Compounds from a Common Fern, Pteris Biaurita
Indian Journal of Experimental Biology Vol. 45, March 2007, pp. 285-290 Characterization of antimicrobial compounds from a common fern, Pteris biaurita A K Dalli, G Saha & U Chakraborty* Plant Biochemistry Laboratory, Department of Botany, University of North Bengal, Siliguri 734013, India Received 29 May 2006; revised 5 December 2006 Methanol extract was prepared from the fronds of Pteris biaurita and partial purification was done by solvent partitioning with diethyl ether and ethyl acetate, followed by hydrolysis and further partitioning with ethyl acetate. The three fractions, thus obtained were bioassayed separately against five test fungi- Curvularia lunata, Fomes lamaoensis, Poria hypobrumea, Fuasrium oxysporum and a bacterium- Bacillus pumilus, by spore germination, radial growth and agar cup techniques. Results revealed that ethyl acetate fraction (III) contained the active principle. TLC plate bioassay of the active fraction revealed inhibition zone at an Rf of 0.5-0.65. Silica gel from this region was scraped, eluted in methanol and subjected to UV-spectrophotometric analysis. An absorption maxima of 278 nm was recorded. HPLC analysis of TLC- eluate revealed a single peak with retention time of 8.1 min. GC-MS analysis revealed six major peaks in the retention time range of 7.2-10.9 min. Comparison with GC-MS libraries revealed that the extracts may contain a mixture of eicosenes and heptadecanes. Keywords: Antimicrobial compounds, Fern, HPLC, GC-MS Pteris biaurita L., is a common fern that grows Bengal. Four of the five fungi were selected for luxuriantly under varying habitats and is used for bioassay as these are common plant pathogenic fungi, ornamental purposes. -

The Pteridaceae Family Diversity in Togo
Biodiversity Data Journal 3: e5078 doi: 10.3897/BDJ.3.e5078 Taxonomic Paper The Pteridaceae family diversity in Togo Komla Elikplim Abotsi‡, Aboudou R. Radji‡, Germinal Rouhan§, Jean-Yves Dubuisson§, Kouami Kokou‡ ‡ Université de Lomé, Lomé, Togo § Museum National d'Histoire Naturelle, Paris cedex 05, France Corresponding author: Komla Elikplim Abotsi ([email protected]) Academic editor: Daniele Cicuzza Received: 10 Apr 2015 | Accepted: 10 Jul 2015 | Published: 15 Jul 2015 Citation: Abotsi K, Radji A, Rouhan G, Dubuisson J, Kokou K (2015) The Pteridaceae family diversity in Togo. Biodiversity Data Journal 3: e5078. doi: 10.3897/BDJ.3.e5078 Abstract Background The Pteridaceae family is the largest fern family in Togo by its specific and generic diversity. Like all other families of ferns in the country, Pteridaceae are poorly studied and has no identification key. The objective of this study is to perform a taxonomic revision and list establishment of this family of leptosporangiate ferns in the light of current available knowledge about the family. Pteridaceae was also assessed in terms of its diversity and conservation status, this was conducted through the recent field data and the existing herbaria specimens. The current study permits to confirm the presence of Pteris similis Kuhn. which brought the number of Pteridaceae to 17 in Togo. New information This study provides first local scientific information about the fern flora of Togo. It confirmed the presence of Pteris similis Kuhn. in Togo and brought the Pteridaceae family diversity to 17 species. A species identification key is provided for the easy identification of the Pteridaceae of Togo.