University of Dundee DOCTOR of MEDICINE the Non-Wnt
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Cell Line P53 Mutation Type UM
A Cell line p53 mutation Type UM-SCC 1 wt UM-SCC5 Exon 5, 157 GTC --> TTC Missense mutation by transversion (Valine --> Phenylalanine UM-SCC6 wt UM-SCC9 wt UM-SCC11A wt UM-SCC11B Exon 7, 242 TGC --> TCC Missense mutation by transversion (Cysteine --> Serine) UM-SCC22A Exon 6, 220 TAT --> TGT Missense mutation by transition (Tyrosine --> Cysteine) UM-SCC22B Exon 6, 220 TAT --> TGT Missense mutation by transition (Tyrosine --> Cysteine) UM-SCC38 Exon 5, 132 AAG --> AAT Missense mutation by transversion (Lysine --> Asparagine) UM-SCC46 Exon 8, 278 CCT --> CGT Missense mutation by transversion (Proline --> Alanine) B 1 Supplementary Methods Cell Lines and Cell Culture A panel of ten established HNSCC cell lines from the University of Michigan series (UM-SCC) was obtained from Dr. T. E. Carey at the University of Michigan, Ann Arbor, MI. The UM-SCC cell lines were derived from eight patients with SCC of the upper aerodigestive tract (supplemental Table 1). Patient age at tumor diagnosis ranged from 37 to 72 years. The cell lines selected were obtained from patients with stage I-IV tumors, distributed among oral, pharyngeal and laryngeal sites. All the patients had aggressive disease, with early recurrence and death within two years of therapy. Cell lines established from single isolates of a patient specimen are designated by a numeric designation, and where isolates from two time points or anatomical sites were obtained, the designation includes an alphabetical suffix (i.e., "A" or "B"). The cell lines were maintained in Eagle's minimal essential media supplemented with 10% fetal bovine serum and penicillin/streptomycin. -
BSCB Newsletter 2017D
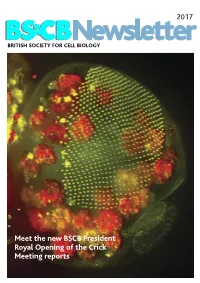
2017 BSCB Newsletter BRITISH SOCIETY FOR CELL BIOLOGY Meet the new BSCB President Royal Opening of the Crick Meeting reports 2017 CONTENTS BSCB Newsletter News 2 Book reviews 7 Features 8 Meeting Reports 24 Summer students 30 Society Business 33 Editorial Welcome to the 2017 BSCB newsletter. After several meeting hosted several well received events for our Front cover: years of excellent service, Kate Nobes has stepped PhD and Postdoc members, which we discuss on The head of a Drosophila pupa. The developing down and handed the reins over to me. I’ve enjoyed page 5. Our PhD and Postdoc reps are working hard compound eye (green) is putting together this years’ newsletter. It’s been great to make the event bigger and better for next year! The composed of several hundred simple units called ommatidia to hear what our members have been up to, and I social events were well attended including the now arranged in an extremely hope you will enjoy reading it. infamous annual “Pub Quiz” and disco after the regular array. The giant conference dinner. Members will be relieved to know polyploidy cells of the fat body (red), the fly equivalent of the The 2016 BSCB/DB spring meeting, organised by our we aren’t including any photos from that here. mammalian liver and adipose committee members Buzz Baum (UCL), Silke tissue, occupy a big area of the Robatzek and Steve Royle, had a particular focus on In this issue, we highlight the great work the BSCB head. Cells and Tissue Architecture, Growth & Cell Division, has been doing to engage young scientists. -

Analysis of Mouse Keratin 6A Regulatory Sequences in Transgenic Mice Reveals Constitutive, Tissue-Speci®C Expression by a Keratin 6A Minigene
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Analysis of Mouse Keratin 6a Regulatory Sequences in Transgenic Mice Reveals Constitutive, Tissue-Speci®c Expression by a Keratin 6a Minigene Donna Mahony, Seetha Karunaratne, Graham Cam,* and Joseph A. Rothnagel Department of Biochemistry and the Institute for Molecular Bioscience, University of Queensland, Brisbane, Queensland, and *Division of Animal Production, CSIRO, Blacktown, New South Wales, Australia The analysis of keratin 6 expression is complicated atin 6 expressing tissues, including the hair follicle, by the presence of multiple isoforms that are tongue, footpad, and nail bed, showing that both expressed constitutively in a number of internal stra- transgenes retained keratinocyte-speci®c expression. ti®ed epithelia, in palmoplantar epidermis, and in Quantitative analysis of b-galactosidase activity veri- the companion cell layer of the hair follicle. In addi- ®ed that both the 1.3 and 0.12 kb keratin 6a promo- tion, keratin 6 expression is inducible in interfollicu- ter constructs produced similar levels of the reporter. lar epidermis and the outer root sheath of the Notably, bothconstructs were constitutively follicle, in response to wounding stimuli, phorbol expressed in the outer root sheath and interfollicular esters, or retinoic acid. In order to establishthecriti- epidermis in the absence of any activating stimulus, cal regions involved in the regulation of keratin 6a suggesting that they lack the regulatory elements (the dominant isoform in mice), we generated trans- that normally silence transcription in these cells. This genic mice withtwo different-sized mouse keratin 6a study has revealed that a keratin 6a minigene con- constructs containing either 1.3 kb or 0.12 kb of 5¢ tains critical cis elements that mediate tissue-speci®c ¯anking sequence linked to the lacZ reporter gene. -

Feedback Interactions Between Cell–Cell Adherens Junctions and Cytoskeletal Dynamics in Newt Lung Epithelial Cells□V Clare M
Molecular Biology of the Cell Vol. 11, 2471–2483, July 2000 Feedback Interactions between Cell–Cell Adherens Junctions and Cytoskeletal Dynamics in Newt Lung Epithelial Cells□V Clare M. Waterman-Storer,*†‡ Wendy C. Salmon,†‡ and E.D. Salmon‡ *Department of Cell Biology and Institute for Childhood and Neglected Diseases, The Scripps Research Institute, La Jolla, California 92037; and ‡Department of Biology, University of North Carolina, Chapel Hill, North Carolina 27599 Submitted February 3, 2000; Revised April 20, 2000; Accepted May 11, 2000 Monitoring Editor: Jennifer Lippincott-Schwartz To test how cell–cell contacts regulate microtubule (MT) and actin cytoskeletal dynamics, we examined dynamics in cells that were contacted on all sides with neighboring cells in an epithelial cell sheet that was undergoing migration as a wound-healing response. Dynamics were recorded using time-lapse digital fluorescence microscopy of microinjected, labeled tubulin and actin. In fully contacted cells, most MT plus ends were quiescent; exhibiting only brief excursions of growth and shortening and spending 87.4% of their time in pause. This contrasts MTs in the lamella of migrating cells at the noncontacted leading edge of the sheet in which MTs exhibit dynamic instability. In the contacted rear and side edges of these migrating cells, a majority of MTs were also quiescent, indicating that cell–cell contacts may locally regulate MT dynamics. Using photoactivation of fluorescence techniques to mark MTs, we found that MTs in fully contacted cells did not undergo retrograde flow toward the cell center, such as occurs at the leading edge of motile cells. Time-lapse fluorescent speckle microscopy of fluorescently labeled actin in fully contacted cells revealed that actin did not flow rearward as occurs in the leading edge lamella of migrating cells. -

Structural and Biochemical Changes Underlying a Keratoderma-Like Phenotype in Mice Lacking Suprabasal AP1 Transcription Factor Function
Citation: Cell Death and Disease (2015) 6, e1647; doi:10.1038/cddis.2015.21 OPEN & 2015 Macmillan Publishers Limited All rights reserved 2041-4889/15 www.nature.com/cddis Structural and biochemical changes underlying a keratoderma-like phenotype in mice lacking suprabasal AP1 transcription factor function EA Rorke*,1, G Adhikary2, CA Young2, RH Rice3, PM Elias4, D Crumrine4, J Meyer4, M Blumenberg5 and RL Eckert2,6,7,8 Epidermal keratinocyte differentiation on the body surface is a carefully choreographed process that leads to assembly of a barrier that is essential for life. Perturbation of keratinocyte differentiation leads to disease. Activator protein 1 (AP1) transcription factors are key controllers of this process. We have shown that inhibiting AP1 transcription factor activity in the suprabasal murine epidermis, by expression of dominant-negative c-jun (TAM67), produces a phenotype type that resembles human keratoderma. However, little is understood regarding the structural and molecular changes that drive this phenotype. In the present study we show that TAM67-positive epidermis displays altered cornified envelope, filaggrin-type keratohyalin granule, keratin filament, desmosome formation and lamellar body secretion leading to reduced barrier integrity. To understand the molecular changes underlying this process, we performed proteomic and RNA array analysis. Proteomic study of the corneocyte cross-linked proteome reveals a reduction in incorporation of cutaneous keratins, filaggrin, filaggrin2, late cornified envelope precursor proteins, hair keratins and hair keratin-associated proteins. This is coupled with increased incorporation of desmosome linker, small proline-rich, S100, transglutaminase and inflammation-associated proteins. Incorporation of most cutaneous keratins (Krt1, Krt5 and Krt10) is reduced, but incorporation of hyperproliferation-associated epidermal keratins (Krt6a, Krt6b and Krt16) is increased. -

Proteomic Expression Profile in Human Temporomandibular Joint
diagnostics Article Proteomic Expression Profile in Human Temporomandibular Joint Dysfunction Andrea Duarte Doetzer 1,*, Roberto Hirochi Herai 1 , Marília Afonso Rabelo Buzalaf 2 and Paula Cristina Trevilatto 1 1 Graduate Program in Health Sciences, School of Medicine, Pontifícia Universidade Católica do Paraná (PUCPR), Curitiba 80215-901, Brazil; [email protected] (R.H.H.); [email protected] (P.C.T.) 2 Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo, Bauru 17012-901, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-41-991-864-747 Abstract: Temporomandibular joint dysfunction (TMD) is a multifactorial condition that impairs human’s health and quality of life. Its etiology is still a challenge due to its complex development and the great number of different conditions it comprises. One of the most common forms of TMD is anterior disc displacement without reduction (DDWoR) and other TMDs with distinct origins are condylar hyperplasia (CH) and mandibular dislocation (MD). Thus, the aim of this study is to identify the protein expression profile of synovial fluid and the temporomandibular joint disc of patients diagnosed with DDWoR, CH and MD. Synovial fluid and a fraction of the temporomandibular joint disc were collected from nine patients diagnosed with DDWoR (n = 3), CH (n = 4) and MD (n = 2). Samples were subjected to label-free nLC-MS/MS for proteomic data extraction, and then bioinformatics analysis were conducted for protein identification and functional annotation. The three Citation: Doetzer, A.D.; Herai, R.H.; TMD conditions showed different protein expression profiles, and novel proteins were identified Buzalaf, M.A.R.; Trevilatto, P.C. -

1 No. Affymetrix ID Gene Symbol Genedescription Gotermsbp Q Value 1. 209351 at KRT14 Keratin 14 Structural Constituent of Cyto
1 Affymetrix Gene Q No. GeneDescription GOTermsBP ID Symbol value structural constituent of cytoskeleton, intermediate 1. 209351_at KRT14 keratin 14 filament, epidermis development <0.01 biological process unknown, S100 calcium binding calcium ion binding, cellular 2. 204268_at S100A2 protein A2 component unknown <0.01 regulation of progression through cell cycle, extracellular space, cytoplasm, cell proliferation, protein kinase C inhibitor activity, protein domain specific 3. 33323_r_at SFN stratifin/14-3-3σ binding <0.01 regulation of progression through cell cycle, extracellular space, cytoplasm, cell proliferation, protein kinase C inhibitor activity, protein domain specific 4. 33322_i_at SFN stratifin/14-3-3σ binding <0.01 structural constituent of cytoskeleton, intermediate 5. 201820_at KRT5 keratin 5 filament, epidermis development <0.01 structural constituent of cytoskeleton, intermediate 6. 209125_at KRT6A keratin 6A filament, ectoderm development <0.01 regulation of progression through cell cycle, extracellular space, cytoplasm, cell proliferation, protein kinase C inhibitor activity, protein domain specific 7. 209260_at SFN stratifin/14-3-3σ binding <0.01 structural constituent of cytoskeleton, intermediate 8. 213680_at KRT6B keratin 6B filament, ectoderm development <0.01 receptor activity, cytosol, integral to plasma membrane, cell surface receptor linked signal transduction, sensory perception, tumor-associated calcium visual perception, cell 9. 202286_s_at TACSTD2 signal transducer 2 proliferation, membrane <0.01 structural constituent of cytoskeleton, cytoskeleton, intermediate filament, cell-cell adherens junction, epidermis 10. 200606_at DSP desmoplakin development <0.01 lectin, galactoside- sugar binding, extracellular binding, soluble, 7 space, nucleus, apoptosis, 11. 206400_at LGALS7 (galectin 7) heterophilic cell adhesion <0.01 2 S100 calcium binding calcium ion binding, epidermis 12. 205916_at S100A7 protein A7 (psoriasin 1) development <0.01 S100 calcium binding protein A8 (calgranulin calcium ion binding, extracellular 13. -

Identification of Endogenous Adenomatous Polyposis Coli Interaction Partners 1 and Β-Catenin-Independent Targets by Proteomics
Author Manuscript Published OnlineFirst on June 3, 2019; DOI: 10.1158/1541-7786.MCR-18-1154 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. 1 Identification of endogenous Adenomatous polyposis coli interaction partners 2 and E-catenin-independent targets by proteomics 3 4 Olesja Popow1,2, João A. Paulo2, Michael H. Tatham3, Melanie S. Volk5, Alejandro 5 Rojas-Fernandez4, Nicolas Loyer5, Ian P. Newton5, Jens Januschke5, Kevin M. 6 Haigis1,6, Inke Näthke5* 7 8 1Cancer Research Institute and Department of Medicine, Beth Israel Deaconess 9 Medical Center, Boston, MA 02215, United States 10 2Department of Cell Biology, Harvard Medical School, Boston, MA 02115, United States 11 3Centre for Gene Regulation and Expression, School of Life Sciences, University of 12 Dundee, Dundee, DD1 5EH, Scotland UK 13 4Center for Interdisciplinary Studies on the Nervous System (CISNe) and Institute of 14 Medicine, Universidad Austral de Chile, Valdivia, Chile 15 5Cell and Developmental Biology, School of Life Sciences, University of Dundee, 16 Dundee, DD1 5EH, Scotland UK 17 6Harvard Digestive Disease Center, Harvard Medical School, Boston, MA 02215, United 18 States 19 20 Running title: The APC interactome and its E-catenin-independent targets. 21 22 Keywords: Adenomatous polyposis coli, destruction complex, colorectal cancer, 23 proteomics, Misshapen-like kinase 1. 1 Downloaded from mcr.aacrjournals.org on October 3, 2021. © 2019 American Association for Cancer Research. Author Manuscript Published OnlineFirst on June 3, 2019; DOI: 10.1158/1541-7786.MCR-18-1154 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. -
BSCB Newsletter 2019A:BSCB Aut2k7
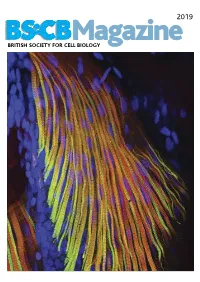
2019 BSCB Magazine BRITISH SOCIETY FOR CELL BIOLOGY 2019 CONTENTS BSCB Magazine News 2 Book reviews 8 Features 9 Meeting Reports 21 Summer students 25 Society Business 32 Editorial Front cover: microscopic Welcome to the 2019 BSCB Magazine! This year Mustafa Aydogan (University of Oxford), as well as to structure of pectoral fin and Susana and Stephen are filling in for our Newsletter BSCB postdoc poster of the year winners Dr Anna hypaxial muscles of a zebrafish Editor Ann Wheeler. We hope you will enjoy this Caballe (University of Oxford) and Dr Agata Gluszek- Danio rerio larvae at four days year’s magazine! Kustusz (University of Edinburgh). post fertilization. The immunostaining highlights the This year we had a number of fantastic one day In 2019, we will have our jointly BSCB-BSDB organization of fast (red) and meetings sponsored by BSCB. These focus meetings Spring meeting at Warwick University from 7th–10th slow (green) myosins. All nuclei are great way to meet and discuss your science with April, organised by BSCB members Susana Godinho are highlighted in blue (hoechst). experts in your field and to strengthen your network of and Vicky Sanz-Moreno. The programme for this collaborators within the UK. You can read more about meeting, which usually provides a broad spectrum of these meetings in the magazine. If you have an idea themes, has a focus on cancer biology: cell for a focus one day meeting, check how to apply for migration/invasion, organelle biogenesis, trafficking, funding on page 4. Our ambassadors have also been cell-cell communication. -

Keratin 6A Gene Silencing Suppresses Cell Invasion and Metastasis of Nasopharyngeal Carcinoma Via the Β‑Catenin Cascade
MOLECULAR MEDICINE REPORTS 19: 3477-3484, 2019 Keratin 6A gene silencing suppresses cell invasion and metastasis of nasopharyngeal carcinoma via the β‑catenin cascade CHUANJUN CHEN1 and HUIGUO SHAN2 1Oncology Department, Xinchang People's Hospital, Shaoxing, Zhejiang 312500; 2Oncology Department, The Affiliated Dongtai Hospital of Nantong University, Dongtai, Jiangsu 224200, P.R. China Received June 4, 2018; Accepted March 1, 2019 DOI: 10.3892/mmr.2019.10055 Abstract. Nasopharyngeal carcinoma (NPC) is a type of head Introduction and neck cancer. This study aimed to study the mechanisms of ectopic keratin 6A (KRT6A) in NPC. Reverse transcrip- Nasopharyngeal carcinoma (NPC) is a type of head and neck tion-quantitative polymerase chain reaction (RT-qPCR) and cancer (1,2). The incidence of NPC is the highest near the parts western blotting were performed to detect KRT6A levels in of or in the ear, nose and throat malignancies. The incidence of NPC cell lines (C666-1, 5-8F and SUNE-1) and a nasopha- NPC has obvious regional clusters and certain ethnic groups ryngeal epithelial cell line (NP69, as a control). After SUNE-1 are likely to experience a higher incidence of NPC than NPC cells had been silenced by KRT6A, cell viability, metas- others. Incidence is low in most areas of the world, generally tasis and invasion were determined using Cell Counting Kit-8, below 1/105 (3). However, in China NPC is mainly distributed wound healing and Transwell assays, respectively. KRT6A in southern China and Southeast Asia (4). The onset ages of levels, metastasis-associated factors and the Wnt/β-catenin NPC are mostly between 40-60 years old, with males having a pathway were measured using RT-qPCR and western blot- higher incidence compared with their female counterparts (5). -

University of Dundee Inke Näthke Sedwick, C.; Nathke, Inke
University of Dundee Inke Näthke Sedwick, C.; Nathke, Inke Published in: Journal of Cell Biology DOI: 10.1083/jcb.1895pi Publication date: 2010 Document Version Publisher's PDF, also known as Version of record Link to publication in Discovery Research Portal Citation for published version (APA): Sedwick, C., & Nathke, I. (2010). Inke Näthke: The ABCs of APC. Journal of Cell Biology, 189(5), 774-775. https://doi.org/10.1083/jcb.1895pi General rights Copyright and moral rights for the publications made accessible in Discovery Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from Discovery Research Portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain. • You may freely distribute the URL identifying the publication in the public portal. Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 01. Oct. 2021 Published May 31, 2010 People & Ideas Inke Näthke: The ABCs of APC Näthke investigates the many functions of adenomatous polyposis coli protein and its contribution to human disease. ighty percent of human colon can- I wanted to go to a foreign country, to cers carry a mutation in adenoma- experience a different way of life, and E tous polyposis coli (APC) protein. -

Cell Structure & Function
Cell Structure & Function Antibodies and Reagents BioLegend is ISO 13485:2016 Certified Toll-Free Tel: (US & Canada): 1.877.BIOLEGEND (246.5343) Tel: 858.768.5800 biolegend.com 02-0012-03 World-Class Quality | Superior Customer Support | Outstanding Value Table of Contents Introduction ....................................................................................................................................................................................3 Cell Biology Antibody Validation .............................................................................................................................................4 Cell Structure/ Organelles ..........................................................................................................................................................8 Cell Development and Differentiation ................................................................................................................................10 Growth Factors and Receptors ...............................................................................................................................................12 Cell Proliferation, Growth, and Viability...............................................................................................................................14 Cell Cycle ........................................................................................................................................................................................16 Cell Signaling ................................................................................................................................................................................18