Epidemiology and Laboratory Diagnosis of Paragonimiasis T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebral Paragonimiasis
CEREBRAL PARAGONIMIASIS REPORT OF FOUR CASES MAJOR SUN KEUN KIM, M.C.* 3rd Army Hospital (Republic of Korea), Pusan, Korea (Received for publication October 6, 1954) ARAGONIMIASIS, or infestation by the lung fluke Paragonimus wester- p manii, is known to be endemic in certain areas of the Far East, par- ticularly in Korea, Japan, and Formosa. In Korea it is found particu- larly in the areas of Yong Hung, Jun Joo, and the Yak Dong river valley. It is acquired by man by the ingestion of raw fresh water fish, an old tradi- tional delicacy in this part of the world. The disease is so common that it is referred to as "To-Zil," or endemic hemoptysis, and it presents a great problem from the standpoint of medical treatment, public health control and national economy. It is known that Paragonimus westermanii may involve the lungs, pleura, liver, intestinal wall, mesenteric lymph glands, testes, muscles, peritoneum, and brain. The following 4 cases of cerebral cysts caused by paragonimiasis, which were encountered during the last year, are reported because of the rarity of cerebral involvement. CASE REPORTS Case 1. An 8-year-old Korean lad was admitted because of motor aphasia, right hemiparesis, and right 7th nerve paresis. He had a history of epileptiform seizures every 2-3 weeks from the age of 8 to 6 years, at which time the seizures stopped but there was insidious onset of right-sided hemiparesis. Examination. He was a somewhat drowsy boy in no acute distress. His face was slightly puffy. His mentality was low. -

Report of the WHO Expert Consultation on Foodborne Trematode Infections and Taeniasis/Cysticercosis
Report of the WHO Expert Consultation on Foodborne Trematode Infections and Taeniasis/Cysticercosis Vientiane, Lao People's Democratic Republic 12-16 October 2009 © World Health Organization 2011 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. -

Infographic Paragonimiasis
Foodborne parasitic infections PARAGONIMIASIS (Lung fluke) Paragonimiasis, or lung fluke The most common species Paragonimus spp. is a common disease, is caused by infection in Asia are P. westermani, parasite of crustacean-eating with several species of trematodes P. heterotremus and mammals such as dogs, cats, ? belonging to the genus P. philippinensis. tigers, mongooses and monkeys. Introduction Paragonimus. (reservoir definitive hosts). The adult flukes live in the lungs of infected mammals and lay eggs that are coughed up through the airways and either expectorated in the sputum or swallowed and Transmission defecated. and risk When the eggs reach freshwater, they develop Eating into miracidia that will inhabit various species undercooked factors infected of aquatic snails where they will asexually freshwater reproduce and eventually give rise to more crustaceans Lack of developed larvae called cercariae. Definitive host sanitation (Fluke) and open The cercariae will then further inhabit various defecation freshwater crustaceans, which act as second intermediate hosts, such as crabs and nd 2 intermediate Free living crayfish but also shrimp and even frogs. host When the crustaceans are eaten raw or undercooked, the metacercariae that is the infective stage for several mammals will excyst 1st intermediate in the intestine and penetrate their way through the intestinal wall, peritoneum, diaphragm Free living host and pleura - eventually reaching the lungs and completing the cycle. The incubation period is 65 to 90 days. When worms reach the lungs, symptoms Triclabendazole and praziquantel are both in humans may include chronic cough with WHO-recommended medicines for treatment of blood-stained sputum, chest pain with difficult paragonimiasis in humans. -

Meeting Report
Meeting Report EXPERT CONSULTATION TO ACCELERATE CONTROL OF FOODBORNE TREMATODE INFECTIONS, TAENIASIS AND CYSTICERCOSIS 17–19 May 2017 Seoul, Republic of Korea WORLD HEALTH ORGANIZATION REGIONAL OFFICE FOR THE WESTERN PACIFIC RS/2017/GE/35(KOR) English only MEETING REPORT EXPERT CONSULTATION TO ACCELERATE CONTROL OF FOODBORNE TREMATODE INFECTIONS, TAENIASIS AND CYSTICERCOSIS Convened by: WORLD HEALTH ORGANIZATION REGIONAL OFFICE FOR THE WESTERN PACIFIC Seoul, Republic of Korea 17–19 May 2017 Not for sale Printed and distributed by: World Health Organization Regional Office for the Western Pacific Manila, Philippines December 2017 NOTE The views expressed in this report are those of the participants of the Expert Consultation to Accelerate Control of Foodborne Trematode Infections, Taeniasis and Cysticercosis and do not necessarily reflect the policies of the conveners. This report has been prepared by the World Health Organization Regional Office for the Western Pacific for Member States in the Region and for those who participated in the Expert Consultation to Accelerate Control of Foodborne Trematode Infections, Taeniasis and Cysticercosis in Seoul, Republic of Korea from 17 to 19 May 2017. CONTENTS ABBREVIATIONS SUMMARY 1. INTRODUCTION ............................................................................................................................................. 1 1.1 Meeting organization ............................................................................................................................ 1 1.2 Meeting -

Early Serodiagnosis of Trichinellosis by ELISA Using Excretory–Secretory
Sun et al. Parasites & Vectors (2015) 8:484 DOI 10.1186/s13071-015-1094-9 RESEARCH Open Access Early serodiagnosis of trichinellosis by ELISA using excretory–secretory antigens of Trichinella spiralis adult worms Ge-Ge Sun, Zhong-Quan Wang*, Chun-Ying Liu, Peng Jiang, Ruo-Dan Liu, Hui Wen, Xin Qi, Li Wang and Jing Cui* Abstract Background: The excretory–secretory (ES) antigens of Trichinella spiralis muscle larvae (ML) are the most commonly used diagnostic antigens for trichinellosis. Their main disadvantage for the detection of anti-Trichinella IgG is false-negative results during the early stage of infection. Additionally, there is an obvious window between clinical symptoms and positive serology. Methods: ELISA with adult worm (AW) ES antigens was used to detect anti-Trichinella IgG in the sera of experimentally infected mice and patients with trichinellosis. The sensitivity and specificity were compared with ELISAs with AW crude antigens and ML ES antigens. Results: In mice infected with 100 ML, anti-Trichinella IgG were first detected by ELISA with the AW ES antigens, crude antigens and ML ES antigens 8, 12 and 12 days post-infection (dpi), respectively. In mice infected with 500 ML, specific antibodies were first detected by ELISA with the three antigen preparations at 10, 8 and 10 dpi, respectively. The sensitivity of the ELISA with the three antigen preparations for the detection of sera from patients with trichinellosis at 35 dpi was 100 %. However, when the patients’ sera were collected at 19 dpi, the sensitivities of the ELISAs with the three antigen preparations were 100 % (20/20), 100 % (20/20) and 75 % (15/20), respectively (P < 0.05). -

Evaluation of Igg4 Subclass Antibody Detection by Peptide-Based ELISA for the Diagnosis of Human Paragonimiasis Heterotrema
ISSN (Print) 0023-4001 ISSN (Online) 1738-0006 Korean J Parasitol Vol. 51, No. 6: 763-766, December 2013 ▣ BRIEF COMMUNICATION http://dx.doi.org/10.3347/kjp.2013.51.6.763 Evaluation of IgG4 Subclass Antibody Detection by Peptide-Based ELISA for the Diagnosis of Human Paragonimiasis Heterotrema 1, 1 1 1 2 Pewpan M. Intapan *, Oranuch Sanpool , Penchom Janwan , Porntip Laummaunwai , Nimit Morakote , Yoon Kong3 and Wanchai Maleewong1 1Department of Parasitology and Research and Diagnostic Center for Emerging Infectious Diseases, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand; 2Department of Parasitology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand; 3Department of Molecular Parasitology, Sungkyunkwan University School of Medicine and Center for Molecular Medicine, Samsung Biomedical Research Institute, Suwon 440-746, Korea Abstract: A synthetic peptide was prepared based on the antigenic region of Paragonimus westermani pre-procathepsin L, and its applicability for immunodiagnosis for human paragonimiasis (due to Paragonimus heterotremus) was tested us- ing an ELISA to detect IgG4 antibodies in the sera of patients. Sera from other helminthiases, tuberculosis, and healthy volunteers were used as the references. This peptide-based assay system gave sensitivity, specificity, accuracy, and posi- tive and negative predictive values of 100%, 94.6%, 96.2%, 100%, and 88.9%, respectively. Cross reactivity was fre- quently seen against the sera of fascioliasis (75%) and hookworm infections (50%). Since differential diagnosis between paragonimiasis and fascioliasis can be easily done by clinical presentation and fascioliasis serology, this cross reaction is not a serious problem. Sera from patients with other parasitoses (0-25%) rarely responded to this synthetic antigen. -

Praziquantel Treatment in Trematode and Cestode Infections: an Update
Review Article Infection & http://dx.doi.org/10.3947/ic.2013.45.1.32 Infect Chemother 2013;45(1):32-43 Chemotherapy pISSN 2093-2340 · eISSN 2092-6448 Praziquantel Treatment in Trematode and Cestode Infections: An Update Jong-Yil Chai Department of Parasitology and Tropical Medicine, Seoul National University College of Medicine, Seoul, Korea Status and emerging issues in the use of praziquantel for treatment of human trematode and cestode infections are briefly reviewed. Since praziquantel was first introduced as a broadspectrum anthelmintic in 1975, innumerable articles describ- ing its successful use in the treatment of the majority of human-infecting trematodes and cestodes have been published. The target trematode and cestode diseases include schistosomiasis, clonorchiasis and opisthorchiasis, paragonimiasis, het- erophyidiasis, echinostomiasis, fasciolopsiasis, neodiplostomiasis, gymnophalloidiasis, taeniases, diphyllobothriasis, hyme- nolepiasis, and cysticercosis. However, Fasciola hepatica and Fasciola gigantica infections are refractory to praziquantel, for which triclabendazole, an alternative drug, is necessary. In addition, larval cestode infections, particularly hydatid disease and sparganosis, are not successfully treated by praziquantel. The precise mechanism of action of praziquantel is still poorly understood. There are also emerging problems with praziquantel treatment, which include the appearance of drug resis- tance in the treatment of Schistosoma mansoni and possibly Schistosoma japonicum, along with allergic or hypersensitivity -

Eosinophilia in Returning Travellers and Migrants from the Tropics: UK Recommendations for Investigation and Initial Management
Journal of Infection (2010) 60,1e20 www.elsevierhealth.com/journals/jinf CLINICAL GUIDELINES OF THE BRITISH INFECTION SOCIETY Eosinophilia in returning travellers and migrants from the tropics: UK recommendations for investigation and initial management Anna M. Checkley a,*, Peter L. Chiodini a, David H. Dockrell b, Imelda Bates c, Guy E. Thwaites a, Helen L. Booth d, Michael Brown a, Stephen G. Wright a, Alison D. Grant a, David C. Mabey a, Christopher J.M. Whitty a, Frances Sanderson e, On behalf of the British Infection Society and The Hospital for Tropical Diseases a Hospital for Tropical Diseases, Capper Street, London WC1E 6JB, UK b Section of Infection, Inflammation and Immunity, University of Sheffield, School of Medicine and Biomedical Sciences, Royal Hallamshire Hospital, Glossop Road, Sheffield S10 2JF, UK c Liverpool School of Tropical Medicine, Pembroke Place, Liverpool L3 5QA, UK d University College London Hospitals NHS Trust, 235 Euston Road, London NW1 2BU, UK e Imperial College Healthcare NHS Trust, Charing Cross Hospital, Fulham Palace Road, London W6 8RF, UK Accepted 13 November 2009 KEYWORDS Summary Eosinophilia is a common finding in returning travellers and migrants, and in this Eosinophilia; group it often indicates an underlying helminth infection. Infections are frequently either Helminth; asymptomatic or associated with non-specific symptoms, but some can cause severe disease. Traveller; Here the British Infection Society guidelines group reviews common and serious infectious Migrant; causes of eosinophilia, and outlines a scheme for investigating returning travellers and mi- Investigation; grants. All returning travellers and migrants with eosinophilia should be investigated with con- Management centrated stool microscopy and strongyloides serology, in addition to tests specific to the region they have visited. -

Report of the WHO Informal Meeting on Use of Triclabendazole in Fascioliasis Control
Report of the WHO Informal Meeting on use of triclabendazole in fascioliasis control WHO headquarters, Geneva, Switzerland 17–18 October 2006 WHO/CDS/NTD/PCT/2007.1 Report of the WHO Informal Meeting on use of triclabendazole in fascioliasis control WHO headquarters, Geneva, Switzerland 17–18 October 2006 © World Health Organization 2007 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. -
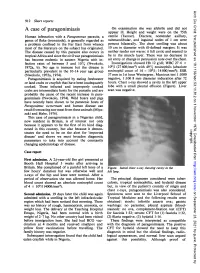
A Case of Paragonimiasis on Examination She Was Afebrile and Did Not Appear Ill
Arch Dis Child: first published as 10.1136/adc.53.11.912 on 1 November 1978. Downloaded from 912 Short reports A case of paragonimiasis On examination she was afebrile and did not appear ill. Height and weight were on the 75th Human infestation with a Paragonimus parasite, a centile (Tanner). Discrete, nontender axillary, genus of fluke (trematode), is generally regarded as submandibular, and inguinal nodes of 1 cm were a problem confined to the Far East from whence present bilaterally. The chest swelling was about most of the literature on the subject has originated. 10 cm in diameter with ill-defined margins. It was The disease caused by this parasite also occurs in neither tender nor warm: it felt cystic and seemed to tropical Africa and since the civil war paragonimiasis be in the muscle layer. There was no decrease in has become endemic in eastern Nigeria with in- air entry or change in percussion note over the chest. fection rates of between 5 and 10 % (Nwokolo, Investigations showed Hb 12 g/dl; WBC 27.6 x 1972a, b). No age is immune but the disease is 109/l (27 600/mm3) with 49% eosinophils (absolute particularly prevalent in the 10-14 year age group eosinophil count of 14 x 109/l; 14000/mm3); ESR (Nwokolo, 1972a, 1974). 37 mm in 1st hour Westergren; Mantoux test 1:1000 Paragonimiasis is acquired by eating freshwater negative, 1:100 8 mm diameter induration after 72 or land crabs or crayfish that have been inadequately hours. Chest x-ray showed a cavity in the left upper cooked. -

Neglected Tropical Diseases - Dracunculiasis (Guinea-Worm Disease)
WEEKLY EPIDEMIOLOGICAL REPORT A publication of the Epidemiology Unit Ministry of Health & Indigenous Medical Services 231, de Saram Place, Colombo 01000, Sri Lanka Tele: + 94 11 2695112, Fax: +94 11 2696583, E mail: [email protected] Epidemiologist: +94 11 2681548, E mail: [email protected] Web: http://www.epid.gov.lk Vol. 47 No. 33 08th– 14th Aug 2020 Neglected Tropical Diseases - Dracunculiasis (Guinea-worm disease) Dracunculiasis (Guinea-worm disease) Dracunculiasis is a parasitic illness at the brink of eradication, with only 54 cases reported in 2019. These 54 were reported from 4 African countries. The parasite enters the human body through ingestion of water contaminated with fleas that are infected with the parasite. The water fleas are killed in the stomach, liberating which interprets as they acquire infection in the the larvae inside them. These larvae penetrate same way as other intermediate hosts but are the intestine wall and migrate within tissues, and not involved in transmitting the infection to the in the process grow to their full size (60-100cm). definitive host. The disease is transmitted to The fertilized adult female worm emerges from humans through ingestion of food, water or soil its exit –usually a lower limb, where it forms a contaminated with the parasite eggs, or after painful blister. When infected persons immerse direct contact with animal hosts. There are 4 their legs in water to soothe the pain, the worm forms of Echinococcosis, each caused by a dif- releases its larvae into the water. These larvae ferent organism of the genus Echinococcus: are ingested by tiny crustaceans known as water Form Causative organism fleas, where they mature into their infective cystic echinococ- stage. -

Paragonimiasisadded Jan 2016
Paragonimiasis Paragonimiasis added Jan 2016 BASIC EPIDEMIOLOGY Infectious Agent Paragonimus species, a parasitic lung fluke (flat worm). More than 30 species of trematodes (flukes) of the genus Paragonimus have been reported which infect animals and humans; the most important is P. westermani, which occurs primarily in Asia. Although rare, human paragonimiasis from P. kellicotti has been acquired in the United States. Transmission Transmission occurs through consumption of raw, salted, pickled, or partially cooked freshwater crabs or crayfish (crawfish) containing infectious larvae (metacercariae). The larvae are released when the crab or crayfish is digested and they migrate within the body, most often ending up in the lungs. Infection can also be acquired by ingestion of raw meat from other infected vertebrae hosts that contain young flukes (e.g., wild boars). Transmission has also been implicated from contaminated utensils, such as knives or cutting boards. Infection is not transmitted directly from person to person. Incubation Period Variable; approximately 7-12 weeks after ingestion of the infectious larvae (when flukes mature and begin to lay eggs). The long, variable, poorly defined interval until symptoms appear depends on the organ invaded and the number of worms involved. Communicability Eggs may be discharged by those infected for up to 20 years. Duration of infection in mollusk and crustacean hosts is not well defined. Animals, such as pigs, dogs and a variety of feline species, can also harbor P. westermani. Clinical Illness Disease most frequently involves the lungs as adult flukes living in the lung cause lung disease. Initial signs and symptoms may be diarrhea and abdominal pain followed several days later by fever, chest pain, and fatigue.