Tyrosine Phosphatase Assay System Instructions for Use of Product V2471
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ATP-Induced Focal Adhesion Kinase Activity Is Negatively Modulated by Phospholipase D2 in PC12 Cells
EXPERIMENTAL and MOLECULAR MEDICINE, Vol. 33, No. 3, 150-155, September 2001 ATP-induced focal adhesion kinase activity is negatively modulated by phospholipase D2 in PC12 cells Yoe-Sik Bae1 and Sung Ho Ryu1,2 Introduction 1 Division of Molecular and Life Sciences, Pohang University of Purinergic receptors have been reported to play impor- Science and Technology, Pohang 790-784, Korea tant roles on the regulation of neuronal cell functions 2 Corresponding author: Tel, +82-54-279-2292; (Communi et al., 2000; Di Iorio et al., 1998). ATP, a Fax, +82-54-279-2199; E-mail, [email protected] ligand for the receptors modulate various cellular re- sponses such as mitogenic and morphogenic activity in Accepted 18 September 2001 PC12 rat pheochromocytoma cells (Neary et al., 1996; Soltoff et al., 1998; Schindelholz et al., 2000). Stimu- Abbreviations: Fak, focal adhesion kinase; PLD, phospholipase D; lation of cells with ATP induces tyrosine phosphorylation PA, phosphatidic acid; PC, phosphatidylcholine; DAG, diacylglyc- of several cytoskeletal proteins and focal adhesion erol; PBt, phosphatidylbutanol; PKC, protein kinase C; PAP, phos- molecules such as focal adhesion kinase (Fak), proline- phatidic acid phosphohydrolase rich tyrosine kinase (Pyk2), and paxillin (Soltoff et al., 1998; Schindelholz et al., 2000). Since these cytosk- eleton-associated proteins have been regarded as important factors for the regulation of neuronal cell Abstract functions, the study on the regulatory mechanism for the proteins remains an important issue. Extracellular ATP has been known to modulate vari- Phospholipase D (PLD) catalyzes the hydrolysis of ous cellular responses including mitogenesis, secre- phosphatidylcholine (PC) into phosphatidic acid (PA) tion and morphogenic activity in neuronal cells. -

Datasheet for Protein Phosphatase 1 (PP1) (P0754; Lot 0121306)
Supplied in: 200 mM NaCl, 50 mM HEPES Unit Definition: One unit is defined as Notes On Use: Avoid freeze/thaw cycles. Can be Protein the amount of enzyme that hydrolyzes 1 nmol of stored for 1 week or less at –20°C. (pH 7.0 @ 25°C), 1 mM MnCl2, 0.1 mM EGTA, Phosphatase 1 2.5 mM dithiothreitol, 0.025% Tween-20 and p-Nitrophenyl Phosphate (50 mM) (NEB #P0757) 50% glycerol. Store at –70°C in 1 minute at 30°C in a total reaction volume of The following information can be used as (PP1) 50 µl. suggested initial conditions for dephosphorylation 1-800-632-7799 Applications: PP1 can be used to release of proteins with PP1. [email protected] phosphate groups from phosphorylated serine, Specific Activity: ~ 80,000 units/mg. www.neb.com 0.1 unit of PP1 removes ~100% of phosphates P0754S 012130614061 threonine and tyrosine residues in proteins. Note that different proteins are dephosphorylated at Molecular Weight: 37.5 kDa. (0.5 nmol) from phosphoserine/threonine different rates. residues in phosphorylase a as well as in P0754S r y Purity: PP1 has been purified to > 90% phosphorylated myelin basic protein (phospho- 100 units 2,500 U/ml Lot: 0121306 Reagents Supplied with Enzyme: homogeneity as determined by SDS-PAGE and MyBP, 18.5 kDa) in 30 minutes in a 50 µl reaction. RECOMBINANT Store at –70°C Exp: 6/14 10X NEBuffer for Protein MetalloPhosphatases Coomassie Blue staining. The concentration of phospho-MyBP is 10 µM (PMP) with respect to phosphate. Description: Protein Phosphatase 1 (PP1) is a 10X MnCl2 (10 mM) Quality Assurance: PP1 contains no detectable Mn2+-dependent protein phosphatase with activity protease activity. -

Calmodulin-Dependent Protein Kinase II–Protein Phosphatase 1 Switch Facilitates Specificity in Postsynaptic Calcium Signaling
An ultrasensitive Ca2؉͞calmodulin-dependent protein kinase II–protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signaling J. Michael Bradshaw*†‡, Yoshi Kubota*, Tobias Meyer†, and Howard Schulman* Departments of *Neurobiology and †Molecular Pharmacology, Stanford University School of Medicine, Stanford, CA 94305 Edited by Roger A. Nicoll, University of California, San Francisco, CA, and approved July 21, 2003 (received for review May 7, 2003) The strength of hippocampal synapses can be persistently in- hence would provide a distinct Ca2ϩ activation region for creased by signals that activate Ca2؉͞calmodulin-dependent pro- CaMKII compared with other Ca2ϩ-activated enzymes at the tein kinase II (CaMKII). This CaMKII-dependent long-term potenti- synapse. In fact, it has been hypothesized that the signaling ation is important for hippocampal learning and memory. In this network controlling CaMKII autophosphorylation is a bistable work we show that CaMKII exhibits an intriguing switch-like type of switch that allows CaMKII to remain autophosphory- activation that likely is important for changes in synaptic strength. lated long after Ca2ϩ returns to a basal level (16). We found that autophosphorylation of CaMKII by itself showed a In this work we demonstrate experimentally that CaMKII 2؉ Ϸ ϩ steep dependence on Ca concentration [Hill coefficient (nH) 5]. responds in a switch-like fashion to Ca2 : CaMKII transitions 2؉ Ϸ However, an even steeper Ca dependence (nH 8) was observed rapidly from little to near-total autophosphorylation over a when autophosphorylation is balanced by the dephosphorylation narrow range of Ca2ϩ. Interestingly, this switch-like response was activity of protein phosphatase 1 (PP1). -

Role of Phospholipases in Adrenal Steroidogenesis
229 1 W B BOLLAG Phospholipases in adrenal 229:1 R29–R41 Review steroidogenesis Role of phospholipases in adrenal steroidogenesis Wendy B Bollag Correspondence should be addressed Charlie Norwood VA Medical Center, One Freedom Way, Augusta, GA, USA to W B Bollag Department of Physiology, Medical College of Georgia, Augusta University (formerly Georgia Regents Email University), Augusta, GA, USA [email protected] Abstract Phospholipases are lipid-metabolizing enzymes that hydrolyze phospholipids. In some Key Words cases, their activity results in remodeling of lipids and/or allows the synthesis of other f adrenal cortex lipids. In other cases, however, and of interest to the topic of adrenal steroidogenesis, f angiotensin phospholipases produce second messengers that modify the function of a cell. In this f intracellular signaling review, the enzymatic reactions, products, and effectors of three phospholipases, f phospholipids phospholipase C, phospholipase D, and phospholipase A2, are discussed. Although f signal transduction much data have been obtained concerning the role of phospholipases C and D in regulating adrenal steroid hormone production, there are still many gaps in our knowledge. Furthermore, little is known about the involvement of phospholipase A2, Endocrinology perhaps, in part, because this enzyme comprises a large family of related enzymes of that are differentially regulated and with different functions. This review presents the evidence supporting the role of each of these phospholipases in steroidogenesis in the Journal Journal of Endocrinology adrenal cortex. (2016) 229, R1–R13 Introduction associated GTP-binding protein exchanges a bound GDP for a GTP. The G protein with GTP bound can then Phospholipids serve a structural function in the cell in that activate the enzyme, phospholipase C (PLC), that cleaves they form the lipid bilayer that maintains cell integrity. -

(PDE 1 B 1) Correlates with Brain Regions Having Extensive Dopaminergic Innervation
The Journal of Neuroscience, March 1994, 14(3): 1251-l 261 Expression of a Calmodulin-dependent Phosphodiesterase lsoform (PDE 1 B 1) Correlates with Brain Regions Having Extensive Dopaminergic Innervation Joseph W. Polli and Randall L. Kincaid Section on Immunology, Laboratory of Molecular and Cellular Neurobiology, National Institute on Alcohol Abuse and Alcoholism, Rockville, Maryland 20852 Cyclic nucleotide-dependent protein phosphorylation plays PDE implies an important physiological role for Ca2+-regu- a central role in neuronal signal transduction. Neurotrans- lated attenuation of CAMP-dependent signaling pathways mitter-elicited increases in cAMP/cGMP brought about by following dopaminergic stimulation. activation of adenylyl and guanylyl cyclases are downre- [Key words: CAMP, cyclase, striatum, dopamine, basal gulated by multiple phosphodiesterase (PDE) enzymes. In ganglia, DARPP-321 brain, the calmodulin (CaM)-dependent isozymes are the major degradative activities and represent a unique point of Cyclic nucleotides, acting as “second messengers”or via direct intersection between the cyclic nucleotide- and calcium effects, regulate a diverse array of neuronal functions, from ion (Ca*+)-mediated second messenger systems. Here we de- channel conductance to gene expression. Hydrolysis of 3’,5’- scribe the distribution of the PDEl Bl (63 kDa) CaM-depen- cyclic nucleotidesto 5’-nucleosidemonophosphates is the major dent PDE in mouse brain. An anti-peptide antiserum to this mechanismfor decreasingintracellular cyclic nucleotide levels. isoform immunoprecipitated -3O-40% of cytosolic PDE ac- This reaction is catalyzed by cyclic nucleotide phosphodiester- tivity, whereas antiserum to PDElA2 (61 kDa isoform) re- ase (PDE) enzymes that constitute a large superfamily (Beavo moved 60-70%, demonstrating that these isoforms are the and Reifsynder, 1990). -

Compartment-Specific Phosphorylation of Phosducin In
Faculty Scholarship 2007 Compartment-specific hoP sphorylation of Phosducin in Rods Underlies Adaptation to Various Levels of Illumination Hongman Song Marycharmain Belcastro E. J. Young Maxim Sokolov Follow this and additional works at: https://researchrepository.wvu.edu/faculty_publications Digital Commons Citation Song, Hongman; Belcastro, Marycharmain; Young, E. J.; and Sokolov, Maxim, "Compartment-specific hospP horylation of Phosducin in Rods Underlies Adaptation to Various Levels of Illumination" (2007). Faculty Scholarship. 92. https://researchrepository.wvu.edu/faculty_publications/92 This Article is brought to you for free and open access by The Research Repository @ WVU. It has been accepted for inclusion in Faculty Scholarship by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 32, pp. 23613–23621, August 10, 2007 © 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Compartment-specific Phosphorylation of Phosducin in Rods Underlies Adaptation to Various Levels of Illumination* Received for publication, March 7, 2007, and in revised form, June 11, 2007 Published, JBC Papers in Press, June 14, 2007, DOI 10.1074/jbc.M701974200 Hongman Song, Marycharmain Belcastro, E. J. Young, and Maxim Sokolov1 From the Departments of Ophthalmology and Biochemistry, West Virginia University School of Medicine and West Virginia University Eye Institute, Morgantown, West Virginia 26506 Phosducin is a major phosphoprotein of rod photoreceptors specific complex with the ␥ subunits of visual heterotrimeric that interacts with the G␥ subunits of heterotrimeric G pro- G protein, transducin (4, 5), and other heterotrimeric G pro- teins in its dephosphorylated state. -

Serum 5-Nucleotidase by Theodore F
J Clin Pathol: first published as 10.1136/jcp.7.4.341 on 1 November 1954. Downloaded from J. clin. Path. (1954), 7, 341. SERUM 5-NUCLEOTIDASE BY THEODORE F. DIXON AND MARY PURDOM t0o From the Biochemistry Department, the Institute of Orthopaedics, Stanmore, Middlesex (RECEIVED FOR PUBLICATION DECEMBER 8. 1953) Reis (1934, 1940, 1951) has demonstrated the add the molybdate solution, and dilute with washings to presence of alkaline phosphatase in various tissues 1 litre with water. specifically hydrolysing 5-nucleotidase such as Standard Phosphate Solution.-KH2PO4 0.02195 g., adenosine and inosine-5-phosphoric acids. The and 50 g. trichloracetic acid, made up to 1 litre. This enzyme has its optimum action at pH 7.8 and in all solution contains 0.02 mg. P in 4 ml. human tissues except intestinal mucosa its activity, at the physiological pH range, is much more Methods pronounced than that of the non-specific alkaline Non-specific alkaline phosphatase activity is high at phosphatase. Thus ossifying cartilage, one of the pH 9 towards phenylphosphate adenosine phosphate classical sites of high alkaline phosphatase activity, and glycerophosphate, in this decreasing order (cf. Reis, although very active against say phenyl phosphoric 1951). At pH 7.5, however, the total phosphatase acids at pH 9, is more active against adenosine-5- activity is equally low towards phenyl- or glycero-phos- phosphoric at pH 7.5. This finding suggested to phate -nd the higher activity towards adenosine-5- phosphate at this reaction is reasonably inferred to be Reis that the enzyme might play a part in calcifica- due to the specific 5-nucleotidase. -

Regulation of Calmodulin-Stimulated Cyclic Nucleotide Phosphodiesterase (PDE1): Review
95-105 5/6/06 13:44 Page 95 INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 18: 95-105, 2006 95 Regulation of calmodulin-stimulated cyclic nucleotide phosphodiesterase (PDE1): Review RAJENDRA K. SHARMA, SHANKAR B. DAS, ASHAKUMARY LAKSHMIKUTTYAMMA, PONNIAH SELVAKUMAR and ANURAAG SHRIVASTAV Department of Pathology and Laboratory Medicine, College of Medicine, University of Saskatchewan, Cancer Research Division, Saskatchewan Cancer Agency, 20 Campus Drive, Saskatoon SK S7N 4H4, Canada Received January 16, 2006; Accepted March 13, 2006 Abstract. The response of living cells to change in cell 6. Differential inhibition of PDE1 isozymes and its environment depends on the action of second messenger therapeutic applications molecules. The two second messenger molecules cAMP and 7. Role of proteolysis in regulating PDE1A2 Ca2+ regulate a large number of eukaryotic cellular events. 8. Role of PDE1A1 in ischemic-reperfused heart Calmodulin-stimulated cyclic nucleotide phosphodiesterase 9. Conclusion (PDE1) is one of the key enzymes involved in the complex interaction between cAMP and Ca2+ second messenger systems. Some PDE1 isozymes have similar kinetic and 1. Introduction immunological properties but are differentially regulated by Ca2+ and calmodulin. Accumulating evidence suggests that the A variety of cellular activities are regulated through mech- activity of PDE1 is selectively regulated by cross-talk between anisms controlling the level of cyclic nucleotides. These Ca2+ and cAMP signalling pathways. These isozymes are mechanisms include synthesis, degradation, efflux and seque- also further distinguished by various pharmacological agents. stration of cyclic adenosine 3':5'-monophosphate (cAMP) and We have demonstrated a potentially novel regulation of PDE1 cyclic guanosine 3':5'- monophosphate (cGMP) within the by calpain. -

The Protein Phosphatase PP2A Plays Multiple Roles in Plant Development by Regulation of Vesicle Traffic—Facts and Questions
International Journal of Molecular Sciences Review The Protein Phosphatase PP2A Plays Multiple Roles in Plant Development by Regulation of Vesicle Traffic—Facts and Questions Csaba Máthé *, Márta M-Hamvas, Csongor Freytag and Tamás Garda Department of Botany, Faculty of Science and Technology, University of Debrecen, H-4032 Debrecen, Hungary; [email protected] (M.M.-H.); [email protected] (C.F.); [email protected] (T.G.) * Correspondence: [email protected] Abstract: The protein phosphatase PP2A is essential for the control of integrated eukaryotic cell functioning. Several cellular and developmental events, e.g., plant growth regulator (PGR) mediated signaling pathways are regulated by reversible phosphorylation of vesicle traffic proteins. Reviewing present knowledge on the relevant role of PP2A is timely. We discuss three aspects: (1) PP2A regulates microtubule-mediated vesicle delivery during cell plate assembly. PP2A dephosphorylates members of the microtubule associated protein family MAP65, promoting their binding to microtubules. Regulation of phosphatase activity leads to changes in microtubule organization, which affects vesicle traffic towards cell plate and vesicle fusion to build the new cell wall between dividing cells. (2) PP2A-mediated inhibition of target of rapamycin complex (TORC) dependent signaling pathways contributes to autophagy and this has possible connections to the brassinosteroid signaling pathway. (3) Transcytosis of vesicles transporting PIN auxin efflux carriers. PP2A regulates vesicle localization and recycling of PINs related to GNOM (a GTP–GDP exchange factor) mediated pathways. The proper intracellular traffic of PINs is essential for auxin distribution in the plant body, thus in whole Citation: Máthé, C.; M-Hamvas, M.; plant development. -

G Protein Regulation of MAPK Networks
Oncogene (2007) 26, 3122–3142 & 2007 Nature Publishing Group All rights reserved 0950-9232/07 $30.00 www.nature.com/onc REVIEW G Protein regulation of MAPK networks ZG Goldsmith and DN Dhanasekaran Fels Institute for Cancer Research and Molecular Biology, Temple University School of Medicine, Philadelphia, PA, USA G proteins provide signal-coupling mechanisms to hepta- the a-subunits has been used as a basis for the helical cell surface receptors and are criticallyinvolved classification of G proteins into Gs,Gi,Gq and G12 in the regulation of different mitogen-activated protein families in which the a-subunits that show more than kinase (MAPK) networks. The four classes of G proteins, 50% homology are grouped together (Simon et al., defined bythe G s,Gi,Gq and G12 families, regulate 1991). In G-protein-coupled receptor (GPCR)-mediated ERK1/2, JNK, p38MAPK, ERK5 and ERK6 modules by signaling pathways, ligand-activated receptors catalyse different mechanisms. The a- as well as bc-subunits are the exchange of the bound GDP to GTP in the a-subunit involved in the regulation of these MAPK modules in a following which the GTP-bound a-subunit disassociate context-specific manner. While the a- and bc-subunits from the receptor as well as the bg-subunit. The GTP- primarilyregulate the MAPK pathwaysvia their respec- bound a-subunit and the bg-subunit stimulate distinct tive effector-mediated signaling pathways, recent studies downstream effectors including enzymes, ion channels have unraveled several novel signaling intermediates and small GTPase, thus regulating multiple signaling including receptor tyrosine kinases and small GTPases pathways including those involved in the activation of through which these G-protein subunits positivelyas well mitogen-activated protein kinase (MAPK) modules as negativelyregulate specific MAPK modules. -
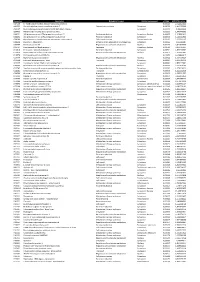
Accession Description Biological Process Cellular Component P-Value SCZ/CTRL Ration A4FU69 EF-Hand Calcium-Binding Domain-Contai
Accession Description Biological Process Cellular component p-Value SCZ/CTRL ration A4FU69 EF-hand calcium-binding domain-containing protein 5 0,001101 2,724427411 A4UGR9 Xin actin-binding repeat-containing protein 2 Cytoskeletal anchoring Cytoplasm 0,006756 1,413953388 A6NCE7 Microtubule-associated proteins 1A/1B light chain 3 beta 2 Cytoplasm 0,001417 1,99612969 B2RPK0 Putative high mobility group protein B1-like 1 0,032314 1,590743352 O00231 26S proteasome non-ATPase regulatory subunit 11 Protein metabolism Cytoplasm; Nucleus 0,000029 1,428664042 O00232 26S proteasome non-ATPase regulatory subunit 12 Protein metabolism Cytoplasm 0,008566 1,544545922 O00264 Membrane-associated progesterone receptor component 1 Cell communication Plasma membrane 0,001459 2,322924147 O00429 Dynamin-1-like protein Mitochondrion organization and biogenesis Cytoplasm 0,006560 0,06391487 O00567 Nucleolar protein 56 Regulation of nucleotide metabolism Nucleus 0,007330 3,842896338 O14737 Programmed cell death protein 5 Apoptosis Cytoplasm; Nucleus 0,006358 3,836727237 O14818 Proteasome subunit alpha type-7 Protein metabolism Cytoplasm 0,030521 1,893928387 O14979 Heterogeneous nuclear ribonucleoprotein D-like Regulation of nucleotide metabolism Nucleus 0,000637 2,150005885 O15078 Centrosomal protein of 290 kDa 0,015359 14,26648619 O15347 High mobility group protein B3 Regulation of nucleotide metabolism Nucleus 0,005500 1,364309014 O15540 Fatty acid-binding protein_ brain Transport Cytoplasm 0,000087 3,125786118 O43237 Cytoplasmic dynein 1 light intermediate -

Analyses of PDE-Regulated Phosphoproteomes Reveal Unique and Specific Camp-Signaling Modules in T Cells
Analyses of PDE-regulated phosphoproteomes reveal unique and specific cAMP-signaling modules in T cells Michael-Claude G. Beltejara, Ho-Tak Laua, Martin G. Golkowskia, Shao-En Onga, and Joseph A. Beavoa,1 aDepartment of Pharmacology, University of Washington, Seattle, WA 98195 Contributed by Joseph A. Beavo, May 28, 2017 (sent for review March 10, 2017; reviewed by Paul M. Epstein, Donald H. Maurice, and Kjetil Tasken) Specific functions for different cyclic nucleotide phosphodiester- to bias T-helper polarization toward Th2, Treg, or Th17 pheno- ases (PDEs) have not yet been identified in most cell types. types (13, 14). In a few cases increased cAMP may even potentiate Conventional approaches to study PDE function typically rely on the T-cell activation signal (15), particularly at early stages of measurements of global cAMP, general increases in cAMP- activation. Recent MS-based proteomic studies have been useful dependent protein kinase (PKA), or the activity of exchange in characterizing changes in the phosphoproteome of T cells under protein activated by cAMP (EPAC). Although newer approaches various stimuli such as T-cell receptor stimulation (16), prosta- using subcellularly targeted FRET reporter sensors have helped glandin signaling (17), and oxidative stress (18), so much of the define more compartmentalized regulation of cAMP, PKA, and total Jurkat phosphoproteome is known. Until now, however, no EPAC, they have limited ability to link this regulation to down- information on the regulation of phosphopeptides by PDEs has stream effector molecules and biological functions. To address this been available in these cells. problem, we have begun to use an unbiased mass spectrometry- Inhibitors of cAMP PDEs are useful tools to study PKA/EPAC- based approach coupled with treatment using PDE isozyme- mediated signaling, and selective inhibitors for each of the 11 PDE – selective inhibitors to characterize the phosphoproteomes of the families have been developed (19 21).