Role of Phospholipases in Adrenal Steroidogenesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ATP-Induced Focal Adhesion Kinase Activity Is Negatively Modulated by Phospholipase D2 in PC12 Cells
EXPERIMENTAL and MOLECULAR MEDICINE, Vol. 33, No. 3, 150-155, September 2001 ATP-induced focal adhesion kinase activity is negatively modulated by phospholipase D2 in PC12 cells Yoe-Sik Bae1 and Sung Ho Ryu1,2 Introduction 1 Division of Molecular and Life Sciences, Pohang University of Purinergic receptors have been reported to play impor- Science and Technology, Pohang 790-784, Korea tant roles on the regulation of neuronal cell functions 2 Corresponding author: Tel, +82-54-279-2292; (Communi et al., 2000; Di Iorio et al., 1998). ATP, a Fax, +82-54-279-2199; E-mail, [email protected] ligand for the receptors modulate various cellular re- sponses such as mitogenic and morphogenic activity in Accepted 18 September 2001 PC12 rat pheochromocytoma cells (Neary et al., 1996; Soltoff et al., 1998; Schindelholz et al., 2000). Stimu- Abbreviations: Fak, focal adhesion kinase; PLD, phospholipase D; lation of cells with ATP induces tyrosine phosphorylation PA, phosphatidic acid; PC, phosphatidylcholine; DAG, diacylglyc- of several cytoskeletal proteins and focal adhesion erol; PBt, phosphatidylbutanol; PKC, protein kinase C; PAP, phos- molecules such as focal adhesion kinase (Fak), proline- phatidic acid phosphohydrolase rich tyrosine kinase (Pyk2), and paxillin (Soltoff et al., 1998; Schindelholz et al., 2000). Since these cytosk- eleton-associated proteins have been regarded as important factors for the regulation of neuronal cell Abstract functions, the study on the regulatory mechanism for the proteins remains an important issue. Extracellular ATP has been known to modulate vari- Phospholipase D (PLD) catalyzes the hydrolysis of ous cellular responses including mitogenesis, secre- phosphatidylcholine (PC) into phosphatidic acid (PA) tion and morphogenic activity in neuronal cells. -

Supplemental Figure 1. Vimentin
Double mutant specific genes Transcript gene_assignment Gene Symbol RefSeq FDR Fold- FDR Fold- FDR Fold- ID (single vs. Change (double Change (double Change wt) (single vs. wt) (double vs. single) (double vs. wt) vs. wt) vs. single) 10485013 BC085239 // 1110051M20Rik // RIKEN cDNA 1110051M20 gene // 2 E1 // 228356 /// NM 1110051M20Ri BC085239 0.164013 -1.38517 0.0345128 -2.24228 0.154535 -1.61877 k 10358717 NM_197990 // 1700025G04Rik // RIKEN cDNA 1700025G04 gene // 1 G2 // 69399 /// BC 1700025G04Rik NM_197990 0.142593 -1.37878 0.0212926 -3.13385 0.093068 -2.27291 10358713 NM_197990 // 1700025G04Rik // RIKEN cDNA 1700025G04 gene // 1 G2 // 69399 1700025G04Rik NM_197990 0.0655213 -1.71563 0.0222468 -2.32498 0.166843 -1.35517 10481312 NM_027283 // 1700026L06Rik // RIKEN cDNA 1700026L06 gene // 2 A3 // 69987 /// EN 1700026L06Rik NM_027283 0.0503754 -1.46385 0.0140999 -2.19537 0.0825609 -1.49972 10351465 BC150846 // 1700084C01Rik // RIKEN cDNA 1700084C01 gene // 1 H3 // 78465 /// NM_ 1700084C01Rik BC150846 0.107391 -1.5916 0.0385418 -2.05801 0.295457 -1.29305 10569654 AK007416 // 1810010D01Rik // RIKEN cDNA 1810010D01 gene // 7 F5 // 381935 /// XR 1810010D01Rik AK007416 0.145576 1.69432 0.0476957 2.51662 0.288571 1.48533 10508883 NM_001083916 // 1810019J16Rik // RIKEN cDNA 1810019J16 gene // 4 D2.3 // 69073 / 1810019J16Rik NM_001083916 0.0533206 1.57139 0.0145433 2.56417 0.0836674 1.63179 10585282 ENSMUST00000050829 // 2010007H06Rik // RIKEN cDNA 2010007H06 gene // --- // 6984 2010007H06Rik ENSMUST00000050829 0.129914 -1.71998 0.0434862 -2.51672 -

Datasheet for Protein Phosphatase 1 (PP1) (P0754; Lot 0121306)
Supplied in: 200 mM NaCl, 50 mM HEPES Unit Definition: One unit is defined as Notes On Use: Avoid freeze/thaw cycles. Can be Protein the amount of enzyme that hydrolyzes 1 nmol of stored for 1 week or less at –20°C. (pH 7.0 @ 25°C), 1 mM MnCl2, 0.1 mM EGTA, Phosphatase 1 2.5 mM dithiothreitol, 0.025% Tween-20 and p-Nitrophenyl Phosphate (50 mM) (NEB #P0757) 50% glycerol. Store at –70°C in 1 minute at 30°C in a total reaction volume of The following information can be used as (PP1) 50 µl. suggested initial conditions for dephosphorylation 1-800-632-7799 Applications: PP1 can be used to release of proteins with PP1. [email protected] phosphate groups from phosphorylated serine, Specific Activity: ~ 80,000 units/mg. www.neb.com 0.1 unit of PP1 removes ~100% of phosphates P0754S 012130614061 threonine and tyrosine residues in proteins. Note that different proteins are dephosphorylated at Molecular Weight: 37.5 kDa. (0.5 nmol) from phosphoserine/threonine different rates. residues in phosphorylase a as well as in P0754S r y Purity: PP1 has been purified to > 90% phosphorylated myelin basic protein (phospho- 100 units 2,500 U/ml Lot: 0121306 Reagents Supplied with Enzyme: homogeneity as determined by SDS-PAGE and MyBP, 18.5 kDa) in 30 minutes in a 50 µl reaction. RECOMBINANT Store at –70°C Exp: 6/14 10X NEBuffer for Protein MetalloPhosphatases Coomassie Blue staining. The concentration of phospho-MyBP is 10 µM (PMP) with respect to phosphate. Description: Protein Phosphatase 1 (PP1) is a 10X MnCl2 (10 mM) Quality Assurance: PP1 contains no detectable Mn2+-dependent protein phosphatase with activity protease activity. -

Novel Allosteric Modulators of the M1 Muscarinic Acetylcholine Receptor Provide New Insights Into M1-Dependent Synaptic Plasticity and Receptor Signaling
Novel allosteric modulators of the M1 muscarinic acetylcholine receptor provide new insights into M1-dependent synaptic plasticity and receptor signaling By Sean P Moran Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Neuroscience December 14, 2019 Nashville, Tennessee Approved: Sachin Patel M.D., Ph.D. Colleen Niswender, Ph.D. Danny Winder, Ph.D. P. Jeffrey Conn, Ph.D. ACKNOWLEDGMENTS Throughout my scientific career, there have been many people instrumental in my scientific journey. I would first like to thank Jeff Conn for providing a very supportive research atmosphere, for always making me laugh, for his insight and constructive criticism in our one-on-one meetings and for occasionally getting my name correct. I would also like to thank my thesis committee of Sachin Patel, Colleen Niswender and Danny Winder for their unwavering support during my time here at Vanderbilt. Additionally, I have very much enjoyed collaborating with Jerri Rook on many projects and would like to thank her for her sage behavioral pharmacology advice over the years. Also, thanks to the many members of the VCNDD including: Zixiu, Craig, Jon Dickerson, Dan Foster, Max, Nicole, Mark and Carrie who provided intellectual contributions, technical training or research support throughout my graduate career. Thank you to the many other members of the VCNDD, past and present, that have contributed to my various projects over the years. Thanks to the better half of Shames (James). I will our miss our long winded, highly pedantic and semantic discussions that occasionally touched on pharmacology topics…and thanks for making me not homeless. -

Edinburgh Research Explorer
Edinburgh Research Explorer International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list Citation for published version: Davenport, AP, Alexander, SPH, Sharman, JL, Pawson, AJ, Benson, HE, Monaghan, AE, Liew, WC, Mpamhanga, CP, Bonner, TI, Neubig, RR, Pin, JP, Spedding, M & Harmar, AJ 2013, 'International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands', Pharmacological reviews, vol. 65, no. 3, pp. 967-86. https://doi.org/10.1124/pr.112.007179 Digital Object Identifier (DOI): 10.1124/pr.112.007179 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Pharmacological reviews Publisher Rights Statement: U.S. Government work not protected by U.S. copyright General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 02. Oct. 2021 1521-0081/65/3/967–986$25.00 http://dx.doi.org/10.1124/pr.112.007179 PHARMACOLOGICAL REVIEWS Pharmacol Rev 65:967–986, July 2013 U.S. -

(4,5) Bisphosphate-Phospholipase C Resynthesis Cycle: Pitps Bridge the ER-PM GAP
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Topological organisation of the phosphatidylinositol (4,5) bisphosphate-phospholipase C resynthesis cycle: PITPs bridge the ER-PM GAP Shamshad Cockcroft and Padinjat Raghu* Dept. of Neuroscience, Physiology and Pharmacology, Division of Biosciences, University College London, London WC1E 6JJ, UK; *National Centre for Biological Sciences, TIFR-GKVK Campus, Bellary Road, Bangalore 560065, India Address correspondence to: Shamshad Cockcroft, University College London UK; Phone: 0044-20-7679-6259; Email: [email protected] Abstract Phospholipase C (PLC) is a receptor-regulated enzyme that hydrolyses phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) at the plasma membrane (PM) triggering three biochemical consequences, the generation of soluble inositol 1,4,5-trisphosphate (IP3), membrane– associated diacylglycerol (DG) and the consumption of plasma membrane PI(4,5)P2. Each of these three signals triggers multiple molecular processes impacting key cellular properties. The activation of PLC also triggers a sequence of biochemical reactions, collectively referred to as the PI(4,5)P2 cycle that culminates in the resynthesis of this lipid. The biochemical intermediates of this cycle and the enzymes that mediate these reactions are topologically distributed across two membrane compartments, the PM and the endoplasmic reticulum (ER). At the plasma membrane, the DG formed during PLC activation is rapidly converted to phosphatidic acid (PA) that needs to be transported to the ER where the machinery for its conversion into PI is localised. Conversely, PI from the ER needs to be rapidly transferred to the plasma membrane where it can be phosphorylated by lipid kinases to regenerate PI(4,5)P2. -

Antibacterial Activity of Ceramide and Ceramide Analogs Against
www.nature.com/scientificreports OPEN Antibacterial activity of ceramide and ceramide analogs against pathogenic Neisseria Received: 10 August 2017 Jérôme Becam1, Tim Walter 2, Anne Burgert3, Jan Schlegel 3, Markus Sauer3, Accepted: 1 December 2017 Jürgen Seibel2 & Alexandra Schubert-Unkmeir1 Published: xx xx xxxx Certain fatty acids and sphingoid bases found at mucosal surfaces are known to have antibacterial activity and are thought to play a more direct role in innate immunity against bacterial infections. Herein, we analysed the antibacterial activity of sphingolipids, including the sphingoid base sphingosine as well as short-chain C6 and long-chain C16-ceramides and azido-functionalized ceramide analogs against pathogenic Neisseriae. Determination of the minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) demonstrated that short-chain ceramides and a ω-azido- functionalized C6-ceramide were active against Neisseria meningitidis and N. gonorrhoeae, whereas they were inactive against Escherichia coli and Staphylococcus aureus. Kinetic assays showed that killing of N. meningitidis occurred within 2 h with ω–azido-C6-ceramide at 1 X the MIC. Of note, at a bactericidal concentration, ω–azido-C6-ceramide had no signifcant toxic efect on host cells. Moreover, lipid uptake and localization was studied by fow cytometry and confocal laser scanning microscopy (CLSM) and revealed a rapid uptake by bacteria within 5 min. CLSM and super-resolution fuorescence imaging by direct stochastic optical reconstruction microscopy demonstrated homogeneous distribution of ceramide analogs in the bacterial membrane. Taken together, these data demonstrate the potent bactericidal activity of sphingosine and synthetic short-chain ceramide analogs against pathogenic Neisseriae. Sphingolipids are composed of a structurally related family of backbones termed sphingoid bases, which are sometimes referred to as ‘long-chain bases’ or ‘sphingosines’. -

Searching for Novel Peptide Hormones in the Human Genome Olivier Mirabeau
Searching for novel peptide hormones in the human genome Olivier Mirabeau To cite this version: Olivier Mirabeau. Searching for novel peptide hormones in the human genome. Life Sciences [q-bio]. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00340710 HAL Id: tel-00340710 https://tel.archives-ouvertes.fr/tel-00340710 Submitted on 21 Nov 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC THESE pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Discipline : Biologie Informatique Ecole Doctorale : Sciences chimiques et biologiques pour la santé Formation doctorale : Biologie-Santé Recherche de nouvelles hormones peptidiques codées par le génome humain par Olivier Mirabeau présentée et soutenue publiquement le 30 janvier 2008 JURY M. Hubert Vaudry Rapporteur M. Jean-Philippe Vert Rapporteur Mme Nadia Rosenthal Examinatrice M. Jean Martinez Président M. Olivier Gascuel Directeur M. Cornelius Gross Examinateur Résumé Résumé Cette thèse porte sur la découverte de gènes humains non caractérisés codant pour des précurseurs à hormones peptidiques. Les hormones peptidiques (PH) ont un rôle important dans la plupart des processus physiologiques du corps humain. -

Synthesis of Lysophospholipids
Molecules 2010, 15, 1354-1377; doi:10.3390/molecules15031354 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Synthesis of Lysophospholipids Paola D’Arrigo 1,2,* and Stefano Servi 1,2 1 Dipartimento di Chimica, Materiali ed Ingegneria Chimica “Giulio Natta”, Politecnico di Milano, Via Mancinelli 7, 20131 Milano, Italy 2 Centro Interuniversitario di Ricerca in Biotecnologie Proteiche "The Protein Factory", Politecnico di Milano and Università degli Studi dell'Insubria, Via Mancinelli 7, 20131 Milano, Italy * Author to whom correspondence should be addressed; E-Mail: paola.d’[email protected]. Received: 17 February 2010; in revised form: 4 March 2010 / Accepted: 5 March 2010 / Published: 8 March 2010 Abstract: New synthetic methods for the preparation of biologically active phospholipids and lysophospholipids (LPLs) are very important in solving problems of membrane–chemistry and biochemistry. Traditionally considered just as second-messenger molecules regulating intracellular signalling pathways, LPLs have recently shown to be involved in many physiological and pathological processes such as inflammation, reproduction, angiogenesis, tumorogenesis, atherosclerosis and nervous system regulation. Elucidation of the mechanistic details involved in the enzymological, cell-biological and membrane-biophysical roles of LPLs relies obviously on the availability of structurally diverse compounds. A variety of chemical and enzymatic routes have been reported in the literature for the synthesis of LPLs: the enzymatic transformation of natural glycerophospholipids (GPLs) using regiospecific enzymes such as phospholipases A1 (PLA1), A2 (PLA2) phospholipase D (PLD) and different lipases, the coupling of enzymatic processes with chemical transformations, the complete chemical synthesis of LPLs starting from glycerol or derivatives. In this review, chemo- enzymatic procedures leading to 1- and 2-LPLs will be described. -

Antibody Response Cell Antigen Receptor Signaling And
Lysophosphatidic Acid Receptor 5 Inhibits B Cell Antigen Receptor Signaling and Antibody Response This information is current as Jiancheng Hu, Shannon K. Oda, Kristin Shotts, Erin E. of September 24, 2021. Donovan, Pamela Strauch, Lindsey M. Pujanauski, Francisco Victorino, Amin Al-Shami, Yuko Fujiwara, Gabor Tigyi, Tamas Oravecz, Roberta Pelanda and Raul M. Torres J Immunol 2014; 193:85-95; Prepublished online 2 June 2014; Downloaded from doi: 10.4049/jimmunol.1300429 http://www.jimmunol.org/content/193/1/85 Supplementary http://www.jimmunol.org/content/suppl/2014/05/31/jimmunol.130042 http://www.jimmunol.org/ Material 9.DCSupplemental References This article cites 63 articles, 17 of which you can access for free at: http://www.jimmunol.org/content/193/1/85.full#ref-list-1 Why The JI? Submit online. by guest on September 24, 2021 • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2014 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Lysophosphatidic Acid Receptor 5 Inhibits B Cell Antigen Receptor Signaling and Antibody Response Jiancheng Hu,*,1,2 Shannon K. -
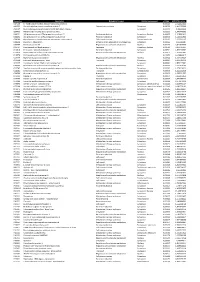
Accession Description Biological Process Cellular Component P-Value SCZ/CTRL Ration A4FU69 EF-Hand Calcium-Binding Domain-Contai
Accession Description Biological Process Cellular component p-Value SCZ/CTRL ration A4FU69 EF-hand calcium-binding domain-containing protein 5 0,001101 2,724427411 A4UGR9 Xin actin-binding repeat-containing protein 2 Cytoskeletal anchoring Cytoplasm 0,006756 1,413953388 A6NCE7 Microtubule-associated proteins 1A/1B light chain 3 beta 2 Cytoplasm 0,001417 1,99612969 B2RPK0 Putative high mobility group protein B1-like 1 0,032314 1,590743352 O00231 26S proteasome non-ATPase regulatory subunit 11 Protein metabolism Cytoplasm; Nucleus 0,000029 1,428664042 O00232 26S proteasome non-ATPase regulatory subunit 12 Protein metabolism Cytoplasm 0,008566 1,544545922 O00264 Membrane-associated progesterone receptor component 1 Cell communication Plasma membrane 0,001459 2,322924147 O00429 Dynamin-1-like protein Mitochondrion organization and biogenesis Cytoplasm 0,006560 0,06391487 O00567 Nucleolar protein 56 Regulation of nucleotide metabolism Nucleus 0,007330 3,842896338 O14737 Programmed cell death protein 5 Apoptosis Cytoplasm; Nucleus 0,006358 3,836727237 O14818 Proteasome subunit alpha type-7 Protein metabolism Cytoplasm 0,030521 1,893928387 O14979 Heterogeneous nuclear ribonucleoprotein D-like Regulation of nucleotide metabolism Nucleus 0,000637 2,150005885 O15078 Centrosomal protein of 290 kDa 0,015359 14,26648619 O15347 High mobility group protein B3 Regulation of nucleotide metabolism Nucleus 0,005500 1,364309014 O15540 Fatty acid-binding protein_ brain Transport Cytoplasm 0,000087 3,125786118 O43237 Cytoplasmic dynein 1 light intermediate -

STA-601-Sphingomyelin-Assay-Kit.Pdf
Introduction Phospholipids are important structural lipids that are the major component of cell membranes and lipid bilayers. Phospholipids contain a hydrophilic head and a hydrophobic tail which give the molecules their unique characteristics. Most phospholipids contain one diglyceride, a phosphate group, and one choline group. Sphingomyelin (ceramide phosphorylcholine) is a sphingolipid found in eukaryotic cell membranes and lipoproteins. Sphingomyelin usually consists of a ceramide and phosphorylcholine molecule where the ceramide core comprises of a fatty acid bonded via an amide bond to a sphingosine molecule. There is a polar head group which is either phosphphoethanolamine or phosphocholine. Sphingomyelin represents about 85% of all sphingolipids and makes up about 10-20% of lipids within the plasma membrane. Sphingomyelin is involved in signal transduction and is highly concentrated in the myelin sheath around many nerve cell axons. The plasma membranes of many cells are rich with sphingomyelin. Sphigolipids are synthesized in a pathway that originates in the ER and is completed in the Golgi apparatus. Many of their functions are done in the plasma membranes and endosomes. Sphingomyelin is converted to ceramide via sphingomyelinases. Ceramides have been implicated in signaling pathways that lead to apoptosis, differentiation and proliferation. Sphingomyelins have been implicated in the pathogenesis of atherosclerosis, inflammation, necrosis, autophagy, senescence, stress response as well as other signaling disease states. Niemann-Pick disease is an inherited disease where deficiency of sphingomyelinase activity results in sphingomyelin accumulating in cells, tissues, and fluids. Other sphingolipid diseases are Fabry disease, Gaucher disease, Tay-Sachs disease, Krabbe disease and Metachromatic leukodystrophy. Cell Biolabs’ Sphingomyelin Assay Kit is a simple fluorometric assay that measures the amount of sphingomyelin present in plasma or serum, tissue homogenates, or cell suspensions in a 96-well microtiter plate format.