Two New Toxic Yellow Inocybe Species from China: Morphological Characteristics, Phylogenetic Analyses and Toxin Detection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
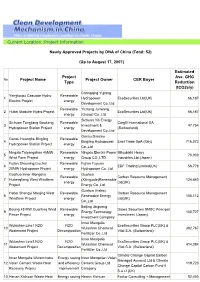
(Total: 52) (Up to August 17, 2007) Project Name P
Current Location :Project Information Newly Approved Projects by DNA of China (Total: 52) (Up to August 17, 2007) Estimated Project Ave. GHG No. Project Name Project Owner CER Buyer Type Reduction (tCO2e/y) Chongqing Yujiang Yangtoupu Cascade Hydro Renewable 1 Hydropower EcoSecurities Ltd(UK) 66,167 Electric Project energy Development Co.,Ltd Renewable Yichang Junwang 2 Hubei Maduhe Hydro Project EcoSecurities Ltd(UK) 66,167 energy (Group) Co.,Ltd Sichuan Yili Energy Sichuan Tongjiang Gaokeng Renewable Cargill International SA 3 Investment & 47,754 Hydropower Station Project energy (Switzerland) Development Co.,Ltd Gansu Diantou Gansu Huanghe Bingling Renewable 4 Bingling Hydropower Enel Trade SpA (Italy) 716,073 Hydropower Station Project energy Co.,Ltd Ningxia Taiyangshan 45MW Renewable Ningxia Electric Power Mitsubishi Heavy 5 70,900 Wind Farm Project energy Group CO.,LTD. Industries,Ltd.(Japan) Fujian Shouning Liuchai Renewable Fujian Fuyuan 6 EDF Trading Limited(UK) 58,778 20MW Hydropower Project energy Hydropower Co.,Ltd Guohua Inner Mongolia Guohua Renewable Carbon Resource Management 7 Huitengliang West Windfarm (Xilinguole)Renewable 124,440 energy Ltd(UK) Project Energy Co.,Ltd Guohua (Hebei) Hebei Shangyi Manjing West Renewable Carbon Resource Management 8 Renewable Energy 105,112 Windfarm Project energy Ltd(UK) Co.,Ltd Beijing Jingneng Beijing 48 MW Guanting Wind Renewable Daiwa Securities SMBC Principal 9 Energy Technology 100,727 Power Project energy Investment (Japan) Investment Company Inner Mongolia Wulashan Line1 N2O N2O EcoSecurities Group PLC(UK) & 10 Wulashan Chemical 392,767 Abatement Project Decomposition Vitol S.A. (Switzerland) Fertilizer Co.,Ltd Inner Mongolia Wulashan Line2 N2O N2O EcoSecurities Group PLC(UK) & 11 Wulashan Chemical 414,084 Abatement Project Decomposition Vitol S.A. -

Major Clades of Agaricales: a Multilocus Phylogenetic Overview
Mycologia, 98(6), 2006, pp. 982–995. # 2006 by The Mycological Society of America, Lawrence, KS 66044-8897 Major clades of Agaricales: a multilocus phylogenetic overview P. Brandon Matheny1 Duur K. Aanen Judd M. Curtis Laboratory of Genetics, Arboretumlaan 4, 6703 BD, Biology Department, Clark University, 950 Main Street, Wageningen, The Netherlands Worcester, Massachusetts, 01610 Matthew DeNitis Vale´rie Hofstetter 127 Harrington Way, Worcester, Massachusetts 01604 Department of Biology, Box 90338, Duke University, Durham, North Carolina 27708 Graciela M. Daniele Instituto Multidisciplinario de Biologı´a Vegetal, M. Catherine Aime CONICET-Universidad Nacional de Co´rdoba, Casilla USDA-ARS, Systematic Botany and Mycology de Correo 495, 5000 Co´rdoba, Argentina Laboratory, Room 304, Building 011A, 10300 Baltimore Avenue, Beltsville, Maryland 20705-2350 Dennis E. Desjardin Department of Biology, San Francisco State University, Jean-Marc Moncalvo San Francisco, California 94132 Centre for Biodiversity and Conservation Biology, Royal Ontario Museum and Department of Botany, University Bradley R. Kropp of Toronto, Toronto, Ontario, M5S 2C6 Canada Department of Biology, Utah State University, Logan, Utah 84322 Zai-Wei Ge Zhu-Liang Yang Lorelei L. Norvell Kunming Institute of Botany, Chinese Academy of Pacific Northwest Mycology Service, 6720 NW Skyline Sciences, Kunming 650204, P.R. China Boulevard, Portland, Oregon 97229-1309 Jason C. Slot Andrew Parker Biology Department, Clark University, 950 Main Street, 127 Raven Way, Metaline Falls, Washington 99153- Worcester, Massachusetts, 01609 9720 Joseph F. Ammirati Else C. Vellinga University of Washington, Biology Department, Box Department of Plant and Microbial Biology, 111 355325, Seattle, Washington 98195 Koshland Hall, University of California, Berkeley, California 94720-3102 Timothy J. -

Social Assessment Report
IPP574 v2 World Bank Loan Public Disclosure Authorized Hunan Forest Restoration and Development Project (HFRDP) Social Assessment Report Public Disclosure Authorized Public Disclosure Authorized Hunan Provincial Forest Foreign Fund Project Management Office Public Disclosure Authorized Social Assessment Team of HFRDP March, 2012 Social Assessment Report for Hunan Forest Restoration and Development Project Abbreviations CFB: County Forestry Bureau FC: Forest Cooperative HFRDP: Hunan Forest Restoration and Development Project HH: household HN: Hunan Province PCP: Participatory consultation and planning PFD: Provincial Forestry Department PPMO: Provincial Project Management Office PRA: Participatory Rural Appraisal SA: Social Assessment TFS: Township Forestry Station Social Assessment Report for Hunan Forest Restoration and Development Project ABSTRACT ................................................................................................................... 1 1. PROJECT BACKGROUND.................................................................................... 10 1.1 Project Background ........................................................................................ 10 1.2 Project Objectives .......................................................................................... 10 1.3 Project Components ....................................................................................... 10 2. PROCESS AND METHODS OF SA ...................................................................... 11 2.1 Process .......................................................................................................... -

Into One of the Two Major Cora Clades (Lücking Et Al
Fungal Diversity into one of the two major Cora clades (Lücking et al. 2014). A (2013). Both share the strongly appressed, filamentous thallus closer relative of C. barbulata is the terrestrial C. arachnoidea in which the horizontally oriented fibrils are embedded in a J. E. Hern. & Lücking (Fig. 128a–c), which is grey-brown gelatinous matrix that gives the thallus a strong metallic shim- when fresh and uniformly thinly tomentose on the upper sur- mer. While the phylogenetic distance between D. metallicum face (Lücking et al. 2013). Cora barbulata can be distin- and its sister species, D. gomezianum,isconsiderable(Dal- guished from C. aspera mainly by the coarsely crenulate, Forno et al., in prep.), the morphological differences are mi- undulate lobe margins and the different hymenophore, nor: D. metallicum has a thinner thallus with indistinct medul- forming large, irregularly dispersed patches on the underside. la, the cyanobacterial filaments are broader (likely influenced by the fungus which produces a sheath with more distinctly 217. Dictyonema gomezianum Lücking, Dal-Forno & puzzle-shaped cells), and particularly the associated fungal Lawrey, sp. nov. hyphae are thicker (4–6 μm). Inocybaceae Jülich Index Fungorum number: IF551502; Facesoffungi The family Inocybaceae is a monophyletic lineage number: FoF01050; Fig. 131d–f within Agaricales. It is species rich and has a world- Etymology: Dedicated to the late Dr. Luis Diego Gómez, wide distribution. The species are small to medium prominent Costa Rican botanist, naturalist, and conservation- sized with a brown spore deposit, and most species ist and long-time director of Las Cruces Biological Station. form ectomycorrhiza with a broad range of host trees Holotype: R. -

The Urban Flood Control Project in the Mountainous Area in Hunan Province Loaned by the Asian Development Bank
The Urban Flood Control Project in the Mountainous Area in Hunan Province Loaned by the Asian Development Bank The External Resettlement Monitoring & Assessment Report (Lengshuijiang City, Lianyuan City, Shuangfeng County, Shaoyang City, Shaodong County, Longhui County, Jiangyong County, Xintian County, Jianghua County, Qiyang County, Ningyuan County, Chenzhou City, Zhuzhou City, Liling City, Zhuzhou County and Youxian County) No.1, 2008 Total No. 1 Hunan Water & Electricity Consulting Corporation (HWECC) September, 2008 Approved by: Wang Hengyang Reviewed by: Long Xiachu Prepared by: Long Xiachu, Wei Riwen 2 Contents 1. Introduction 2. Project Outline 2.1 Project Outline 2.2 Resettlement Outline 3. Establishment and Operation of Resettlement Organizations 3.1 Organization Arrangement 3.2 Organization Operation 4. Project Implementation Progress 4.1 Jiangyong County 4.2 Chenzhou City 5. Resettlement Implementation Progress 5.1 Resettlement Implementation Schedule 5.2 Resettlement Policy and Compensation Standards 5.3 Progress of Land Acquisition 5.4 Progress of Resettlement Arrangement 5.5 Removal Progress of Enterprises and Institutions 5.6 Progress of Resettlement Area Construction 5.7 Arrival and Payment of the Resettlement Fund 6. Psychology and Complaint of the Resettled People 6.1 Complaint Channel 6.2 Complaint Procedures 7. Public Participation, Consultation and Information Publicizing 7.1 Jiangyong County 7.2 Chenzhou City 8. Existed Problems and Suggestions 3 1. Introduction The Urban Flood Control Project in the Mountainous -

Chinacoalchem
ChinaCoalChem Monthly Report Issue May. 2019 Copyright 2019 All Rights Reserved. ChinaCoalChem Issue May. 2019 Table of Contents Insight China ................................................................................................................... 4 To analyze the competitive advantages of various material routes for fuel ethanol from six dimensions .............................................................................................................. 4 Could fuel ethanol meet the demand of 10MT in 2020? 6MTA total capacity is closely promoted ....................................................................................................................... 6 Development of China's polybutene industry ............................................................... 7 Policies & Markets ......................................................................................................... 9 Comprehensive Analysis of the Latest Policy Trends in Fuel Ethanol and Ethanol Gasoline ........................................................................................................................ 9 Companies & Projects ................................................................................................... 9 Baofeng Energy Succeeded in SEC A-Stock Listing ................................................... 9 BG Ordos Started Field Construction of 4bnm3/a SNG Project ................................ 10 Datang Duolun Project Created New Monthly Methanol Output Record in Apr ........ 10 Danhua to Acquire & -

Biodiversity of Wood-Decay Fungi in Italy
AperTO - Archivio Istituzionale Open Access dell'Università di Torino Biodiversity of wood-decay fungi in Italy This is the author's manuscript Original Citation: Availability: This version is available http://hdl.handle.net/2318/88396 since 2016-10-06T16:54:39Z Published version: DOI:10.1080/11263504.2011.633114 Terms of use: Open Access Anyone can freely access the full text of works made available as "Open Access". Works made available under a Creative Commons license can be used according to the terms and conditions of said license. Use of all other works requires consent of the right holder (author or publisher) if not exempted from copyright protection by the applicable law. (Article begins on next page) 28 September 2021 This is the author's final version of the contribution published as: A. Saitta; A. Bernicchia; S.P. Gorjón; E. Altobelli; V.M. Granito; C. Losi; D. Lunghini; O. Maggi; G. Medardi; F. Padovan; L. Pecoraro; A. Vizzini; A.M. Persiani. Biodiversity of wood-decay fungi in Italy. PLANT BIOSYSTEMS. 145(4) pp: 958-968. DOI: 10.1080/11263504.2011.633114 The publisher's version is available at: http://www.tandfonline.com/doi/abs/10.1080/11263504.2011.633114 When citing, please refer to the published version. Link to this full text: http://hdl.handle.net/2318/88396 This full text was downloaded from iris - AperTO: https://iris.unito.it/ iris - AperTO University of Turin’s Institutional Research Information System and Open Access Institutional Repository Biodiversity of wood-decay fungi in Italy A. Saitta , A. Bernicchia , S. P. Gorjón , E. -

Chronology of Mass Killings During the Chinese Cultural Revolution (1966-1976) Song Yongyi Thursday 25 August 2011
Chronology of Mass Killings during the Chinese Cultural Revolution (1966-1976) Song Yongyi Thursday 25 August 2011 Stable URL: http://www.massviolence.org/Article?id_article=551 PDF version: http://www.massviolence.org/PdfVersion?id_article=551 http://www.massviolence.org - ISSN 1961-9898 Chronology of Mass Killings during the Chinese Cultural Revolution (1966-1976) Chronology of Mass Killings during the Chinese Cultural Revolution (1966-1976) Song Yongyi The Chinese Cultural Revolution (1966-1976) was a historical tragedy launched by Mao Zedong and the Chinese Communist Party (CCP). It claimed the lives of several million people and inflicted cruel and inhuman treatments on hundreds of million people. However, 40 years after it ended, the total number of victims of the Cultural Revolution and especially the death toll of mass killings still remain a mystery both in China and overseas. For the Chinese communist government, it is a highly classified state secret, although they do maintain statistics for the so-called abnormal death numbers all over China. Nevertheless, the government, realizing that the totalitarian regime and the endless power struggles in the CCP Central Committee (CCP CC) were the root cause of the Cultural Revolution, has consistently discounted the significance of looking back and reflecting on this important period of Chinese history. They even forbid Chinese scholars from studying it independently and discourage overseas scholars from undertaking research on this subject in China. Owing to difficulties that scholars in and outside China encounter in accessing state secrets, the exact figure of the abnormal death has become a recurring debate in the field of China studies. -

World Bank Document
Hunan Integrated Management of Agricultural Land Pollution (P153115) Procurement Plan I. General Public Disclosure Authorized 1. Bank’s approval Date of the procurement Plan [original: June 27, 2017; 1st Revision: August 17, 2017; 2nd Revision: June 8, 2018; 3rd Revision: Aug. 10, 2018; 4th Revision: April 4, 2019; 5th Revision: December 20, 2019; 6th Revision: March 4, 2020; 7th Revision: March 30, 2020] 2. Date of General Procurement Notice: August 16, 2017 3. Period covered by this procurement plan: March 2020 to December 2020 II. Goods, Works, non-consulting services and Community participation in procurement under Component 1. 1. Prior Review Threshold: Procurement Decisions subject to Prior Review by the Bank as Public Disclosure Authorized stated in Appendix 1 to the Guidelines for Procurement: Procurement Method Prior Review Threshold Procurement Method Threshold US$ US$ ICB and LIB (Goods and Non- Greater than or equal to US$ 1. All Consulting Services ) 10 million NCB (Goods and Non-Consulting Greater than or equal to US$ Greater than or equal to 2. Services ) 0.5 million 2 million Greater than or equal to US$ 3. ICB (Works) All 40 million Greater than or equal to US$ Greater than or equal to 4. NCB (Works) 0.5 million 10 million Community participation in to be specified in the 5 No Threshold procurement Public Disclosure Authorized operation manual 2. Prequalification. Bidders for _Not applicable_ shall be prequalified in accordance with the provisions of paragraphs 2.9 and 2.10 of the Guidelines. 3. Proposed Procedures for CDD Components (as per paragraph. 3.17 of the Guidelines: Detailed procedures for community participation are specified in operation manual. -

World Bank Document
Public Disclosure Authorized Public Disclosure Authorized Goods and Works Procurement Plan in 2007 2007 Name of Subproject: Nuisance Free Vegetable, Changsha County ( Review by Issuing of Bid Contract Cost estimate Procurement P- Contract No. Bank BD opening signing Contract Description method Q USD ( RMB (Y/N) Equivalent Y/N) Works Public Disclosure Authorized Public Disclosure Authorized Vegetable processing 800 NCB Hn workshop 800 , Vegetable 300 à à 1 1 à à à quality test room 300 NCB GJP 80 type plastic sheds GJP80 Hn à à 1 1 à à à 42979 42979 m2 Subtotal à à Goods Public Disclosure Authorized Public Disclosure Authorized 1 Public Disclosure Authorized Public Disclosure Authorized Goods and Works Procurement Plan in 2007 2007 Name of Subproject: Nuisance Free Vegetable, Changsha County ( Review by Issuing of Bid Contract Cost estimate Procurement P- Contract No. Bank BD opening signing Contract Description method Q USD ( RMB (Y/N) Equivalent Y/N) Vegetable Test Devices, including: dehumidifiers 4sets, air conditioners 4sets, refrigerators 4sets, fresh- keeping refrigerated cabinets 4sets, ultrasonic cleaners 3sets, centrifuges 3sets, drying cabinets 3sets, stainless steel electrical distillers 6sets, rotary evaporators 4sets, rapid 16 Hn à à 1 1 detector of pesticide residues 6sets, residual pesticide meters 6sets, full automatic thermo wellwash plus 1sets, electro-heating constant temperature cultivators 6sets, 3+ multifunctional vibrators 6sets, precise PH meters 6sets, freezing dryers 6sets, spiral slice vacuum -

Corporate Social Responsibility White Paper
2020 CEIBS CORPORATE SOCIAL RESPONSIBILITY WHITE PAPER FOREWORD The Covid-19 pandemic has brought mounting research teams, as well as alumni associations and com- uncertainties and complexities to the world economy. Our panies. The professors obtained the research presented globalized society faces the challenge of bringing the in the paper through the employment of detailed CSR virus under control while minimizing its impact on the parameters focused on business leaders, employee economy. Economic difficulties substantially heighten the behavior and their relationship to the external environ- urgency for a more equitable and sustainable society. ment. This granular and nuanced form of research is a powerful tool for guiding the healthy development of CSR. At the same time, there is an ever-pressing need to enrich and expand the CSR framework in the context of The five CEIBS alumni companies featured in the social and economic development. CEIBS has incorporat- white paper offer exceptional examples of aligning busi- ed CSR programs into teaching, research, and student/ ness practices with social needs. Their learning-based alumni activities since its inception. The international busi- future-proof business innovations are a powerful demon- ness school jointly founded by the Chinese government stration of how best to bring CSR to the forefront of busi- and the European Union has accelerated knowledge ness activities. These five firms all received the CSR creation and dissemination during the pandemic to sup- Award in April 2019 at the second CEIBS Alumni Corpo- port economic stability and business development. The rate Social Responsibility Award, organized by the CEIBS institution has also served as a key communication chan- Alumni Association. -

World Bank Document
Hunan Integrated Management of Agricultural Land Pollution (P153115) Procurement Plan I. General Public Disclosure Authorized 1. Bank’s approval Date of the procurement Plan [original: June 27, 2017; 1st Revision: August 17, 2017; 2nd Revision: June 8, 2018; 3rd Revision: Aug. 10, 2018] 2. Date of General Procurement Notice: August 16, 2017 3. Period covered by this procurement plan: August 2017 to February 2019 II. Goods, Works, non-consulting services and Community participation in procurement under Component 1. 1. Prior Review Threshold: Procurement Decisions subject to Prior Review by the Bank as stated in Appendix 1 to the Guidelines for Procurement: Procurement Method Prior Review Threshold Procurement Method Threshold US$ US$ Public Disclosure Authorized ICB and LIB (Goods and Non- Greater than or equal to US$ 1. All Consulting Services ) 10 million NCB (Goods and Non-Consulting Greater than or equal to US$ Greater than or equal to 2. Services ) 0.5 million 2 million Greater than or equal to US$ 3. ICB (Works) All 40 million Greater than or equal to US$ Greater than or equal to 4. NCB (Works) 0.5 million 10 million Community participation in to be specified in the 5 No Threshold procurement operation manual Public Disclosure Authorized 2. Prequalification. Bidders for _Not applicable_ shall be prequalified in accordance with the provisions of paragraphs 2.9 and 2.10 of the Guidelines. 3. Proposed Procedures for CDD Components (as per paragraph. 3.17 of the Guidelines: Detailed procedures for community participation are specified in operation manual. 4. Reference to (if any) Project Operational/Procurement Manual: Project Implementation Manual for World Bank Loan Project P153115 has been prepared by Hunan PPMO.