Production and Chemistry of Transactinide Elements - Yuichiro Nagame, Hiromitsu Haba
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Atomic Properties of the Elements
P E R I O D I C T A B L E Group 1 18 IA Atomic Properties of the Elements VIIIA 2 1 S FREQUENTLY USED FUNDAMENTAL PHYSICAL CONSTANTS§ S 1 1/2 Physical Measurement Laboratory www.nist.gov/pml 2 0 1 second = 9 192 631 770 periods of radiation corresponding to the H transition between the two hyperfine levels of the ground state of 133Cs Standard Reference Data www.nist.gov/srd He 1 Hydrogen −1 § For the most accurate Helium speed of light in vacuum c 299 792 458 m s (exact) values of these and 1.008* −34 4.002602 2 1s 2 Planck constant h 6.626 070 x 10 J s ( ħ /2 ) other constants, visit 13 14 15 16 17 1s −19 13.5984 IIA elementary charge e 1.602 177 x 10 C physics.nist.gov/constants IIIA IVA VA VIA VIIA 24.5874 −31 2 1 electron mass me 9.109 384 x 10 kg 2 3 4 3 2 1 3 S1/2 4 S0 2 5 P°1/2 6 P0 7 S3/2° 8 P2 9 P3/2° 10 S0 mec 0.510 999 MeV −27 Solids Li Be proton mass mp 1.672 622 x 10 kg B C N O F Ne 2 Lithium Beryllium fine-structure constant 1/137.035 999 Liquids Boron Carbon Nitrogen Oxygen Fluorine Neon 6.94* 9.0121831 −1 10.81* 12.011* 14.007* 15.999* 18.99840316* 20.1797 Rydberg constant R 10 973 731.569 m 2 2 2 Gases 2 2 2 2 2 2 2 3 2 2 4 2 2 5 2 2 6 15 1s 2s 2p 1s 2s 1s 2s 1s 2s 1s 2s 1s 2s 1s 2s R c 3.289 841 960 x 10 Hz 2p 2p 1s 2s 2p 2p 2p 5.3917 9.3227 Artificially 8.2980 11.2603 14.5341 13.6181 17.4228 21.5645 R hc 13.605 693 eV 2 1 -19 Prepared 2 3 4 3 2 1 11 S1/2 12 S0 electron volt eV 1.602 176 6 x 10 J 13 P1/2° 14 P0 15 S3/2° 16 P2 17 P3/2° 18 S0 −23 −1 Boltzmann constant k 1.380 65 x 10 J K −1 −1 Na Mg molar gas constant -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -
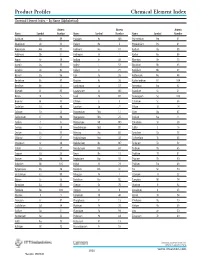
Product Profiles Chemical Element Index
Product Profiles Chemical Element Index Chemical Element Index – By Name (Alphabetical) Atomic Atomic Atomic Name Symbol Number Name Symbol Number Name Symbol Number Actinium Ac 89 Hassium Hs 108 Promethium Pm 61 Aluminum Al 13 Helium He 2 Protactinium Pa 91 Americium Am 95 Holmium Ho 67 Radium Ra 88 Antimony Sb 51 Hydrogen H 1 Radon Rn 86 Argon Ar 18 Indium In 49 Rhenium Re 75 Arsenic As 33 Iodine I 53 Rhodium Rh 45 Astatine At 85 Iridium Ir 77 Rubidium Rb 37 Barium Ba 56 Iron Fe 26 Ruthenium Ru 44 Berkelium Bk 97 Krypton Kr 36 Rutherfordium Rf 104 Beryllium Be 4 Lanthanum La 57 Samarium Sm 62 Bismuth Bi 83 Lawrencium Lr 103 Scandium Sc 21 Boron B 5 Lead Pb 82 Seaborgium Sg 106 Bromine Br 35 Lithium Li 3 Selenium Se 34 Cadmium Cd 48 Lutetium Lu 71 Silicon Si 14 Calcium Ca 20 Magnesium Mg 12 Silver Ag 47 Californium Cf 98 Manganese Mn 25 Sodium Na 11 Carbon C 6 Meitnerium Mt 109 Strontium Sr 38 Cerium Ce 58 Mendelevium Md 101 Sulfur S 16 Cesium Cs 55 Mercury Hg 80 Tantalum Ta 73 Chlorine Cl 17 Molybdenum Mo 42 Technetium Tc 43 Chromium Cr 24 Neilsborium Ns 107 Tellurium Te 52 Cobalt Co 27 Neodymium Nd 60 Terbium Tb 65 Copper Cu 29 Neon Ne 10 Thallium Tl 81 Curium Cm 96 Neptunium Np 93 Thorium Th 90 Dubnium Db 105 Nickel Ni 28 Thulium Tm 69 Dysprosium Dy 66 Niobium Nb 41 Tin Sn 50 Einsteinium Es 99 Nitrogen N 7 Titanium Ti 22 Erbium Er 68 Nobelium No 102 Tungsten W 74 Europium Eu 63 Osmian Os 76 Uranium U 92 Fermium Fm 100 Oxygen O 8 Vanadium V 23 Fluorine F 9 Palladium Pd 46 Xenon Xi 54 Francium Fr 87 Phosphorus P 15 Ytterbium Yb 70 Gadolinium -

Peaceful Berkelium
in your element Peaceful berkelium The first new element produced after the Second World War has led a rather peaceful life since entering the period table — until it became the target of those producing superheavy elements, as Andreas Trabesinger describes. f nomenclature were transitive, element 97 with the finding that in Bk(iii) compounds would be named after an Irish bishop spin–orbit coupling leads to a mixing of the Iwhose philosophical belief was that first excited state and the ground state. This material things do not exist. But between gives rise to unexpected electronic properties the immaterialist George Berkeley and the not present in analogous lanthanide element berkelium stands, of course, the structures containing terbium. Even more fine Californian city of Berkeley (pictured), recently5, a combined experimental and where the element was first produced in computational study revealed that berkelium December 1949. The city became the eponym can have stabilized +iii and +iV oxidation of element 97 “in a manner similar to that states also under mild aqueous conditions, used in naming its chemical homologue indicating a path to separating it from other terbium […] whose name was derived from lanthanides and actinides. the town of Ytterby, Sweden, where the rare The main use of berkelium, however — earth minerals were first found”1. arguably one that wouldn’t have met with A great honour for the city? The mayor Bishop Berkeley’s approval — remains a of Berkeley at the time reportedly displayed PHOTO STOCK / ALAMY AF ARCHIVE distinctly material one: as the target for “a complete lack of interest when he was the synthesis of other transuranium and called with the glad tidings”2. -

Upper Limit of the Periodic Table and the Future Superheavy Elements
CLASSROOM Rajarshi Ghosh Upper Limit of the Periodic Table and the Future Department of Chemistry The University of Burdwan ∗ Superheavy Elements Burdwan 713 104, India. Email: [email protected] Controversy surrounds the isolation and stability of the fu- ture transactinoid elements (after oganesson) in the periodic table. A single conclusion has not yet been drawn for the highest possible atomic number, though there are several the- oretical as well as experimental results regarding this. In this article, the scientific backgrounds of those upcoming super- heavy elements (SHE) and their proposed electronic charac- ters are briefly described. Introduction Totally 118 elements, starting from hydrogen (atomic number 1) to oganesson (atomic number 118) are accommodated in the mod- ern form of the periodic table comprising seven periods and eigh- teen groups. Total 92 natural elements (if technetium is consid- ered as natural) are there in the periodic table (up to uranium hav- ing atomic number 92). In the actinoid series, only four elements— Keywords actinium, thorium, protactinium and uranium—are natural. The Superheavy elements, actinoid rest of the eleven elements—from neptunium (atomic number 93) series, transactinoid elements, periodic table. to lawrencium (atomic number 103)—are synthetic. Elements after actinoids (i.e., from rutherfordium) are called transactinoid elements. These are also called superheavy elements (SHE) as they have very high atomic numbers. Prof. G T Seaborg had Elements after actinoids a very distinct contribution in the field of transuranium element (i.e., from synthesis. For this, Prof. Seaborg was awarded the Nobel Prize in rutherfordium) are called transactinoid elements. 1951. -

Studies of Flerovium and Element 115 Homologs with Macrocyclic Extractants
UNLV Theses, Dissertations, Professional Papers, and Capstones 5-1-2015 Studies of Flerovium and Element 115 Homologs with Macrocyclic Extractants John Dustin Despotopulos University of Nevada, Las Vegas Follow this and additional works at: https://digitalscholarship.unlv.edu/thesesdissertations Part of the Chemistry Commons Repository Citation Despotopulos, John Dustin, "Studies of Flerovium and Element 115 Homologs with Macrocyclic Extractants" (2015). UNLV Theses, Dissertations, Professional Papers, and Capstones. 2345. http://dx.doi.org/10.34917/7645877 This Dissertation is protected by copyright and/or related rights. It has been brought to you by Digital Scholarship@UNLV with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/or on the work itself. This Dissertation has been accepted for inclusion in UNLV Theses, Dissertations, Professional Papers, and Capstones by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. INVESTIGATION OF FLEROVIUM AND ELEMENT 115 HOMOLOGS WITH MACROCYCLIC EXTRACTANTS By John Dustin Despotopulos Bachelor of Science in Chemistry University of Oregon 2010 A dissertation submitted in partial fulfillment of the requirements for the Doctor of Philosophy -

The Chemical Complexities of Plutonium David L
The Chemical Complexities of Plutonium David L. Clark ew people have ever seen plutonium, and far fewer have actually handled or manipu- lated it. Yet this manmade element has arguably altered the course of civilization as Fmuch as copper, bronze, iron, or steel. Within five years of its synthesis, the primary use of plutonium was for the release of nuclear energy in weapons of mass destruction, and it seemed that the new element might lead the human race to the brink of self-annihilation. But instead, plutonium has become a stabilizing agent in global politics, forcing the human race to govern itself without resorting to nuclear war. Never before has a simple chemical element had such a profound impact on the consciousness of mankind. Plutonium has had a similarly humbling impact in the more circumscribed arena of science. Incredibly, it displays physicochemical behaviors that are among the most complex of any element in the periodic table. The pure element exhibits seven distinct crystal phases, is highly reactive, and is known to form compounds, complexes, or alloys with virtually every other element. Molten plutonium is highly corrosive and will slowly react with its container, causing difficulties for handling. When elemental plutonium reacts to give up its valence electrons, it can form a wide variety of positively charged ions with the ability to form up to twelve chemical bonds to other ions or molecules in solution. The element can exhibit five oxidation states, and under certain chemical conditions, four different oxidation states can be present in appreciable amounts simultaneously! No other element displays such a complex chemistry. -

The Elements.Pdf
A Periodic Table of the Elements at Los Alamos National Laboratory Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements A Resource for Elementary, Middle School, and High School Students Click an element for more information: Group** Period 1 18 IA VIIIA 1A 8A 1 2 13 14 15 16 17 2 1 H IIA IIIA IVA VA VIAVIIA He 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 6.941 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 3 Na Mg IIIB IVB VB VIB VIIB ------- VIII IB IIB Al Si P S Cl Ar 22.99 24.31 3B 4B 5B 6B 7B ------- 1B 2B 26.98 28.09 30.97 32.07 35.45 39.95 ------- 8 ------- 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.47 58.69 63.55 65.39 69.72 72.59 74.92 78.96 79.90 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 5 Rb Sr Y Zr NbMo Tc Ru Rh PdAgCd In Sn Sb Te I Xe 85.47 87.62 88.91 91.22 92.91 95.94 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 6 Cs Ba La* Hf Ta W Re Os Ir Pt AuHg Tl Pb Bi Po At Rn 132.9 137.3 138.9 178.5 180.9 183.9 186.2 190.2 190.2 195.1 197.0 200.5 204.4 207.2 209.0 (210) (210) (222) 87 88 89 104 105 106 107 108 109 110 111 112 114 116 118 7 Fr Ra Ac~RfDb Sg Bh Hs Mt --- --- --- --- --- --- (223) (226) (227) (257) (260) (263) (262) (265) (266) () () () () () () http://pearl1.lanl.gov/periodic/ (1 of 3) [5/17/2001 4:06:20 PM] A Periodic Table of the Elements at Los Alamos National Laboratory 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Lanthanide Series* Ce Pr NdPmSm Eu Gd TbDyHo Er TmYbLu 140.1 140.9 144.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Actinide Series~ Th Pa U Np Pu AmCmBk Cf Es FmMdNo Lr 232.0 (231) (238) (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257) ** Groups are noted by 3 notation conventions. -

Synthesis of Superheavy Elements by Cold Fusion
Radiochim. Acta 99, 405–428 (2011) / DOI 10.1524/ract.2011.1854 © by Oldenbourg Wissenschaftsverlag, München Synthesis of superheavy elements by cold fusion By S. Hofmann1,2,∗ 1 GSI Helmholtzzentrum für Schwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany 2 Institut für Kernphysik, Goethe-Universität, Max von Laue-Straße 1, 60438 Frankfurt, Germany (Received February 3, 2011; accepted in revised form April 18, 2011) Superheavy elements / Synthesis / Cold fusion / in 1972, and the RILAC (variable-frequency linear acceler- Cross-sections / Decay properties / Recoil separators / ator) at the RIKEN Nishina Center in Saitama near Tokio in Detector systems 1980. In the middle of the 1960s the concept of the macrosco- pic-microscopic model for calculating binding energies Summary. The new elements from Z = 107 to 112 were of nuclei also at large deformations was invented by synthesized in cold fusion reactions based on targets of lead V. M. Strutinsky [3]. Using this method, a number of the and bismuth. The principle physical concepts are presented measured phenomena could be naturally explained by con- which led to the application of this reaction type in search sidering the change of binding energy as function of defor- experiments for new elements. Described are the technical mation. In particular, it became possible now to calculate the developments from early mechanical devices to experiments with recoil separators. An overview is given of present ex- binding energy of a heavy fissioning nucleus at each point of periments which use cold fusion for systematic studies and the fission path and thus to determine the fission barrier. synthesis of new isotopes. -

Chemistry of Superheavy Elements: New Vistas for Studying Effects of Einstein's Relativity on the Structure of the Periodic Table
Chemistry of superheavy elements: New vistas for studying effects of Einstein's relativity on the structure of the periodic table An international collaboration led by research groups from Mainz and Darmstadt, Germany has achieved the synthesis of a new class of chemical compounds for superheavy elements at the RIKEN Nishina Center for Accelerator-based Research (RNC) in Japan. For the first time, a chemical bond was established between a superheavy element – seaborgium (element 106) in the present study – and a carbon atom. Eighteen atoms of seaborgium were converted into seaborgium hexacarbonyl complexes, which include six carbon monoxide molecules bound to the seaborgium. Its gaseous properties and adsorption to a silicon dioxide surface were studied, and compared with similar compounds of neighbors of seaborgium in the same group of the periodic table. The study opens perspectives for much more detailed investigations of the chemical behavior of elements at the end of the periodic table, where the influence of effects of relativity on chemical properties is most pronounced. Chemical experiments with superheavy elements – with atomic number beyond 104 – are most challenging: First, the very element to be studied has to be artificially created using a particle accelerator. Maximum production rates are on the order of a few atoms per day at most, and are even less for the heavier ones. Second, the atoms decay quickly through radioactive processes – in the present case within about 10 seconds, adding to the experiment's complexity. A strong motivation for such demanding studies is that the very many positively charged protons inside the atomic nuclei accelerate electrons in the atom's shells to very high velocities – about 80 percent of the speed of light. -

Food Periodic Table
Food Chemistry Periodic Table Celebrating the International Year of the Periodic Table 2019 Created by Jane K Parker Acknowledgements and thanks to the RSC Food Group Committee: Robert Cordina, Bryan Hanley, Taichi Inuit, John Points, Kathy Ridgway, Martin Rose, Wendy Russell, Mike Saltmarsh, Maud Silvent, Clive Thomson, Kath Whittaker, Pete Wilde, and to Martin Chadwick, Cian Moloney and Ese Omoaruhke, for contributions to the elements, to Flaticons for use of their free icons, and to Alinea and TDMA for photographs of He and Ti. In slideshow mode, click on an element in periodic table to find out more, return via the RSC Food Group Logo. Contact: [email protected] Food Chemistry Periodic Table H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Ti Pb Bi Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og H Hydrogen 1 Occurrences in food Roles in food • Core element in organic compounds (fats, proteins, • H+ gives one of the five basic tastes – sour. carbohydrates, vitamins). • the higher the concentration of H+, the lower the pH • OH pH 2 Lemon juice (very sour) • H2 H2 H2 H2 H2 pH 3 Apple (sour) C C C C C C • pH 5 Meat (not sour) H3C C C C C C O H H H H H 2 2 2 2 2 • pH 7 Tea or water (not sour) • Hydrogen-bonds, one of the strongest forms of bonding, are crucial for the 3D-structure of many • Key element in water, H2O, which is 70% of the human body and 70% of many foods.