USP Catalog Schedule NOTICE Ensure Accuracy with the New This May–June 2014 Catalog Is the Last Print Edition in 2014
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 8,227,427 B2 Coracci Neto Et Al
USOO8227427B2 (12) United States Patent (10) Patent No.: US 8,227,427 B2 Coracci Neto et al. (45) Date of Patent: Jul. 24, 2012 (54) VETERINARIAN COMPOSITION (52) US. Cl. ........................................ .. 514/27; 514/368 COMPRISING AN ORGANIC SALT 0F (58) Field of Classi?cation Search ................. .. 513/71; LEVAMISOLE IN COMBINATION WITH AT 548/155; 514/27, 368 LEAST ONE AVERMECTIN AND/OR See application ?le for complete search history. MILBEMYCIN (75) Inventors: Dolivar Coracci Neto, Sertaozinho (56) References Cited (BR); Nelson Henriques Fernandes Filho, Jaboticabal (BR); Ricardo da FOREIGN PATENT DOCUMENTS Silva Sercheli, Jaboticabal (BR) BR PI0505716 A 9/2007 GB 2150024 A 6/1985 (73) Assignee: NPA—Nucleo de Pesquisas Aplicadas WO 00/74489 A1 12/2000 Ltda., Jaboticabal (BR) WO 2004/009080 A1 1/2004 OTHER PUBLICATIONS ( * ) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 International Search Report. U.S.C. 154(b) by 575 days. Analytical Report Characterization of Pharmaceutical Input Aurixazol (Feb. 20, 2010). (21) App1.No.: 12/097,683 Primary Examiner * Elli Peselev (22) PCT Filed: Dec. 18, 2006 (74) Attorney, Agent, or Firm * Laurence P. Colton; Smith Risley Tempel Santos LLC (86) PCT No.: PCT/BR2006/000282 § 371 (0X1)’ (57) ABSTRACT (2), (4) Date: Nov. 3, 2008 Veterinarian composition comprising an organic salt of (87) PCT Pub. No.: WO2007/068073 levamisole in combination With at least one avermectin and/ or milbemycin. A veterinarian formulation comprising of organ PCT Pub. Date: Jun. 21, 2007 ics salts of levamisole, more speci?cally to the levamisole salt of 2,6-diiodo-4-nitrophenol and the levamisole salt of 4-hy (65) Prior Publication Data droxy-3-iodo-5-nitrobenzonitrile With avermectins and mil US 2009/0075918 A1 Mar. -

INVESTIGATION of NATURAL PRODUCT SCAFFOLDS for the DEVELOPMENT of OPIOID RECEPTOR LIGANDS by Katherine M
INVESTIGATION OF NATURAL PRODUCT SCAFFOLDS FOR THE DEVELOPMENT OF OPIOID RECEPTOR LIGANDS By Katherine M. Prevatt-Smith Submitted to the graduate degree program in Medicinal Chemistry and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. _________________________________ Chairperson: Dr. Thomas E. Prisinzano _________________________________ Dr. Brian S. J. Blagg _________________________________ Dr. Michael F. Rafferty _________________________________ Dr. Paul R. Hanson _________________________________ Dr. Susan M. Lunte Date Defended: July 18, 2012 The Dissertation Committee for Katherine M. Prevatt-Smith certifies that this is the approved version of the following dissertation: INVESTIGATION OF NATURAL PRODUCT SCAFFOLDS FOR THE DEVELOPMENT OF OPIOID RECEPTOR LIGANDS _________________________________ Chairperson: Dr. Thomas E. Prisinzano Date approved: July 18, 2012 ii ABSTRACT Kappa opioid (KOP) receptors have been suggested as an alternative target to the mu opioid (MOP) receptor for the treatment of pain because KOP activation is associated with fewer negative side-effects (respiratory depression, constipation, tolerance, and dependence). The KOP receptor has also been implicated in several abuse-related effects in the central nervous system (CNS). KOP ligands have been investigated as pharmacotherapies for drug abuse; KOP agonists have been shown to modulate dopamine concentrations in the CNS as well as attenuate the self-administration of cocaine in a variety of species, and KOP antagonists have potential in the treatment of relapse. One drawback of current opioid ligand investigation is that many compounds are based on the morphine scaffold and thus have similar properties, both positive and negative, to the parent molecule. Thus there is increasing need to discover new chemical scaffolds with opioid receptor activity. -

Bulletin Leading the Fight Against Heartworm Disease
BULLETIN LEADING THE FIGHT AGAINST HEARTWORM DISEASE SEPTEMBER HEARTWORM 2017 Q&A VOLUME 44 No. 3 Heartworm History: In What Year Was Heartworm First INSIDE THIS ISSUE Treated? Page 4 From the President Page 8 Research Update Abstracts from the Literature Page 14 Heartworm Hotline: Role of Heat Treatment in Diagnostics Page 19 NEW! Best Practices: Minimizing Heartworm Transmission in Relocated Dogs uestions from members, prac- published in the 1998 AHS Symposium 1 titioners, technicians, and the Proceedings. Dr. Roncalli wrote, “The Page 21 Qgeneral public are often submit- first trial to assess the efficacy of a Welcome Our New AHS ted to the American Heartworm Society microfilaricide (natrium antimonyl tar- Student Liaisons (AHS) via our website. Two of our AHS trate) was conducted some 70 years Board members, Dr. John W.McCall and ago (1927) in Japan by S. Itagaki and R. Page 25 Dr. Tom Nelson, provided the resources Makino.2 Fuadin (stibophen), a trivalent In the News: Surgeons to answer this question: In What Year antimony compound, was tested, intra- Remove a Heartworm from Was Heartworm First Treated? venously, as a microfilaricide by Popescu the Femoral Artery of a Cat The first efforts to treat canine heart- in 1933 in Romania and by W.H. Wright worm disease date back to the 1920s. Dr. and P.C. Underwood in 1934 in the USA. Page 26 Nelson referenced a review article by Dr. In 1949, I.C. Mark evaluated its use Quarterly Update Raffaele Roncalli, “Tracing the History of intraperitoneally.” What’s New From AHS? Heartworms: A 400 Year Perspective,” Continues on page 7 American Heartworm Society / PO Box 8266, Wilmington, DE 19803-8266 Become an American Heartworm Society www.heartwormsociety.org / [email protected] fan on Facebook! Follow us on Twitter! OUR GENEROUS SPONSORS PLATINUM LEVEL PO Box 8266 Wilmington, DE 19803-8266 [email protected] www.heartwormsociety.org Mission Statement The mission of the American Heartworm Society is to lead the vet- erinary profession and the public in the understanding of heartworm disease. -

GAO-18-61, SUNSCREEN: FDA Reviewed Applications For
United States Government Accountability Office Report to Congressional Committees November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed GAO-18-61 November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed Highlights of GAO-18-61, a report to congressional committees Why GAO Did This Study What GAO Found Using sunscreen as directed with other The Food and Drug Administration (FDA), within the Department of Health and sun protective measures may help Human Services, implemented requirements for reviewing applications for reduce the risk of skin cancer—the sunscreen active ingredients within time frames set by the Sunscreen Innovation most common form of cancer in the Act, which was enacted in November 2014. For example, the agency issued a United States. In the United States, guidance document on safety and effectiveness testing in November 2016. sunscreen is considered an over-the- counter drug, which is a drug available As of August 2017, all applications for sunscreen active ingredients remain to consumers without a prescription. pending after the agency determined more safety and effectiveness data are Some sunscreen active ingredients not needed. By February 2015, FDA completed its initial review of the safety and currently marketed in the United States effectiveness data for each of the eight pending applications, as required by the have been available in products in act. FDA concluded that additional data are needed to determine that the other countries for more than a ingredients are generally recognized as safe and effective (GRASE), which is decade. Companies that manufacture needed so that products using the ingredients can subsequently be marketed in some of these ingredients have sought the United States without FDA’s premarket approval. -

Anthem Blue Cross Drug Formulary
Erythromycin/Sulfisoxazole (generic) INTRODUCTION Penicillins ...................................................................... Anthem Blue Cross uses a formulary Amoxicillin (generic) (preferred list of drugs) to help your doctor Amoxicillin/Clavulanate (generic/Augmentin make prescribing decisions. This list of drugs chew/XR) is updated quarterly, by a committee Ampicillin (generic) consisting of doctors and pharmacists, so that Dicloxacillin (generic) the list includes drugs that are safe and Penicillin (generic) effective in the treatment of diseases. If you Quinolones ..................................................................... have any questions about the accessibility of Ciprofloxacin/XR (generic) your medication, please call the phone number Levofloxacin (Levaquin) listed on the back of your Anthem Blue Cross Sulfonamides ................................................................ member identification card. Erythromycin/Sulfisoxazole (generic) In most cases, if your physician has Sulfamethoxazole/Trimethoprim (generic) determined that it is medically necessary for Sulfisoxazole (generic) you to receive a brand name drug or a drug Tetracyclines .................................................................. that is not on our list, your physician may Doxycycline hyclate (generic) indicate “Dispense as Written” or “Do Not Minocycline (generic) Substitute” on your prescription to ensure Tetracycline (generic) access to the medication through our network ANTIFUNGAL AGENTS (ORAL) _________________ of community -

Supplement Ii to the Japanese Pharmacopoeia Fifteenth Edition
SUPPLEMENT II TO THE JAPANESE PHARMACOPOEIA FIFTEENTH EDITION Official From October 1, 2009 English Version THE MINISTRY OF HEALTH, LABOUR AND WELFARE Notice: This English Version of the Japanese Pharmacopoeia is published for the conven- ience of users unfamiliar with the Japanese language. When and if any discrepancy arises between the Japanese original and its English translation, the former is authentic. The Ministry of Health, Labour and Welfare Ministerial Notification No. 425 Pursuant to Paragraph 1, Article 41 of the Pharmaceutical Affairs Law (Law No. 145, 1960), we hereby revise a part of the Japanese Pharmacopoeia (Ministerial Notification No. 285, 2006) as follows*, and the revised Japanese Pharmacopoeia shall come into ef- fect on October 1, 2009. However, in the case of drugs which are listed in the Japanese Pharmacopoeia (hereinafter referred to as “previous Pharmacopoeia”) [limited to those listed in the Japanese Pharmacopoeia whose standards are changed in accordance with this notification (hereinafter referred to as “new Pharmacopoeia”)] and drugs which have been approved as of October 1, 2009 as prescribed under Paragraph 1, Article 14 of the same law [including drugs the Minister of Health, Labour and Welfare specifies (the Ministry of Health and Welfare Ministerial Notification No. 104, 1994) as those ex- empted from marketing approval pursuant to Paragraph 1, Article 14 of the Pharmaceu- tical Affairs Law (hereinafter referred to as “drugs exempted from approval”)], the Name and Standards established in the previous Pharmacopoeia (limited to part of the Name and Standards for the drugs concerned) may be accepted to conform to the Name and Standards established in the new Pharmacopoeia before and on March 31, 2011. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

(12) United States Patent (10) Patent No.: US 9.421,180 B2 Zielinski Et Al
USOO9421 180B2 (12) United States Patent (10) Patent No.: US 9.421,180 B2 Zielinski et al. (45) Date of Patent: Aug. 23, 2016 (54) ANTIOXIDANT COMPOSITIONS FOR 6,203,817 B1 3/2001 Cormier et al. .............. 424/464 TREATMENT OF INFLAMMATION OR 6,323,232 B1 1 1/2001 Keet al. ............ ... 514,408 6,521,668 B2 2/2003 Anderson et al. ..... 514f679 OXIDATIVE DAMAGE 6,572,882 B1 6/2003 Vercauteren et al. ........ 424/451 6,805,873 B2 10/2004 Gaudout et al. ....... ... 424/401 (71) Applicant: Perio Sciences, LLC, Dallas, TX (US) 7,041,322 B2 5/2006 Gaudout et al. .............. 424/765 7,179,841 B2 2/2007 Zielinski et al. .. ... 514,474 (72) Inventors: Jan Zielinski, Vista, CA (US); Thomas 2003/0069302 A1 4/2003 Zielinski ........ ... 514,452 Russell Moon, Dallas, TX (US); 2004/0037860 A1 2/2004 Maillon ...... ... 424/401 Edward P. Allen, Dallas, TX (US) 2004/0091589 A1 5, 2004 Roy et al. ... 426,265 s s 2004/0224004 A1 1 1/2004 Zielinski ..... ... 424/442 2005/0032882 A1 2/2005 Chen ............................. 514,456 (73) Assignee: Perio Sciences, LLC, Dallas, TX (US) 2005, 0137205 A1 6, 2005 Van Breen ..... 514,252.12 2005. O154054 A1 7/2005 Zielinski et al. ............. 514,474 (*) Notice: Subject to any disclaimer, the term of this 2005/0271692 Al 12/2005 Gervasio-Nugent patent is extended or adjusted under 35 et al. ............................. 424/401 2006/0173065 A1 8/2006 BeZwada ...................... 514,419 U.S.C. 154(b) by 19 days. 2006/O193790 A1 8/2006 Doyle et al. -

Sun Lotion Chemicals As Endocrine Disruptors
HORMONES 2015, 14(1):32-46 Review Sun lotion chemicals as endocrine disruptors Sotirios Maipas, Polyxeni Nicolopoulou-Stamati National and Kapodistrian University of Athens, School of Medicine, First Department of Pathology and Cytology Unit, 1st Pathology Laboratory, Athens, Greece Both authors contributed equally to this work ABSTRACT Ultraviolet solar radiation is a well-known environmental health risk factor and the use of sun lotions is encouraged to achieve protection mainly from skin cancer. Sun lotions are cosmetic commercial products that combine active and inactive ingredients and many of these are associated with health problems, including allergic reactions and endocrine disorders. This review focuses on their ability to cause endocrine and reproductive impairments, with empha- sis laid on the active ingredients (common and less common UV filters). In vitro and in vivo studies have demonstrated their ability to show oestrogenic/anti-oestrogenic and androgenic/ anti-androgenic activity. Many ingredients affect the oestrous cycle, spermatogenesis, sexual behaviour, fertility and other reproductive parameters in experimental animals. Their presence in aquatic environments may reveal a new emerging environmental hazard. Key words: Active ingredients, Endocrine disruptors, Environmental hazard, Reproductive impair- ments, Sun creams, Sun lotions, Sunscreens, UV filters 1. INTRODUCTION ing, but the level of photoprotection is insufficient to prevent the harmful effects of UV radiation.3,4 Ultraviolet (UV) solar radiation is one -
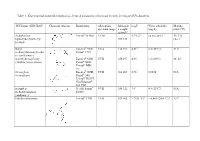
Table 1 - Experimental and Predicted Physical-Chemical Parameters of the Most Recently Investigated UV-Absorbers
Table 1 - Experimental and predicted physical-chemical parameters of the most recently investigated UV-absorbers. INCI name (INN/XAN) Chemical structure Brand name Absorption Molecula LogP Water solubility Melting spectrum range r weight (mg/L) point (°C) (g/mol)4 diethylamino Uvinul® A Plus UVA1 5.7-6.21 <0.01 (20°C) 1 54; 314 hydroxybenzoyl hexyl 397.515 (dec.) 1 benzoate Butyl Eusolex® 9020, UVA 310.393 4.514 2.2 (25°C)4 83.54 methoxydibenzoylmetha Parsol® 1789 ne (avobenzone) 4-methylbenzylidene Eusolex® 6300 UVB 258.397 4.95 1.3 (20°C) 66–68 camphor (enzacamene) Parsol® 5000 Uvinul® MBC 95 Octocrylene Eusolex® OCR, UVB 361.485 6.783 0.00383 N/A (octocrilene) Parsol® 340, Uvinul® N539T, NeoHeliopan® 303 USP isoamyl p- Neo Heliopan® UVB 248.322 3.61 4.9 (25°C)1 N/A methoxycinnamate E1000 (amiloxate) Ethylhexyl triazone Uvinul® T150 UVB 823.092 > 7(20 °C) 6 < 0.001 (20.0 °C) 6 1296 Ethylhexyl Parsol® MCX, UVB 290.403 6.14 0.041 (24 °C and N/A methoxycinnamate Heliopan® New pH 7.1) 4 (octinoxate) Ethylhexyl dimethyl Escalol™ 507 UVB 277.4084 5.774 0.54 (25 °C) 4 N/A PABA (padimate-O) Arlatone 507 Eusolex 6007 benzophenone-3 Eusolex® 4360 UVA2+ UVB 228.247 3.72 3.7 (20°C) 2 62-652 (oxybenzone) bis-ethylhexyloxyphenol Tinosorb® S UVA1+UVB 627.826 12.61 <10-4 80.401 methoxyphenol triazine (bemotrizinol) Phenylbenzimidazole Eusolex® 232 UVA2+ UVB 274.2945 -1.1 (pH 5) > 30% (As sodium N/A sulfonic acid Parsol® HS -2.1 (pH 8)5 or (ensulizole) Neo Heliopan® triethanolammoniu Hydro m salt at 20°C) 5 1 (3) 2 (34) 3 (44) 4 Pubchem 5 SCCP/1056/06 Opinion on phenylbenzimidazole sulfonic acid and its salts 6 BASF safety data sheet Table 2 – In vitro studies for the assessment of skin permeation/penetration of sunscreens. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate.