Epilepsy and Movement Disorders in Inborn Errors of Metabolism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Epilepsy in Childhood
Psychogenic Non-epileptic Seizures Dr Katharina Junejo Consultant Child and Adolescent Psychiatrist Aim Overview of basic principles in childhood epilepsy PNES Diagnosis of epilepsy Every child’s brain is capable of generating seizures with certain pro-convulsive drugs, acute metabolic disturbance, CNS infection, acute head trauma Risk of epilepsy after acute and provoked seizure is 3-5% Emotional stress is not considered provoking factor Henri Gastaut and colleagues proposed a classification of seizures: “all attempts at classification of seizures are hampered by our limited knowledge of the underlying pathological processes within the brain and that any classification must of necessity be a tentative one and will be subject to change with every advance in scientific understanding of epilepsy” (Gastaut et al 1964). Epilepsy has variable clinical presentations Convulsions (tonic- Staring spells clonic seizures) (absences) Period with confusion with or without Variants of automatism (complex paroxysmal events partial seizures) during sleep (common frontal lobe seizures) Limb or body jerk movements (myoclonus) Loss of speech/decline in Spasms (infantile or social, cognitive function Falls or drops epileptic spasms) (Landau-Kleffner (atonic but also syndrome) tonic seizures) ILAE Revised Terminology for Organization of Seizures and Epilepsies 2011 ‐ 2013 Classification of seizures: generalised and focal seizures Electroclinical syndromes and other epilepsies grouped by specificity of diagnosis (arranged by typical age of onset) Major conceptual changes: using aetiological classification of epilepsy-genetic, metabolic, immune, infectious and unknown causes Changes in terminology Operational diagnosis of epilepsy: occurrence of 2 or more unprovoked seizures, irrespective of seizure type (as recurrence risk is 80-90%) Careful history is only diagnostic test as many paroxysmal disorders that mimic epileptic seizures (most common vasovagal syncope or reflex anoxic seizure) Limitations of EEG: approx. -

Prognostic Utility of Hypsarrhythmia Scoring in Children with West
Clinical Neurology and Neurosurgery 184 (2019) 105402 Contents lists available at ScienceDirect Clinical Neurology and Neurosurgery journal homepage: www.elsevier.com/locate/clineuro Prognostic utility of hypsarrhythmia scoring in children with West syndrome T after ketogenic diet ⁎ Yunjian Zhang1, Lifei Yu1, Yuanfeng Zhou, Linmei Zhang, Yi Wang, Shuizhen Zhou Department of Pediatric Neurology, Children’s Hospital of Fudan University, China ARTICLE INFO ABSTRACT Keywords: Objective: The aim of this study was to evaluate the clinical efficacy and electroencephalographic (EEG) changes West syndrome of West syndrome after ketogenic diet (KD) therapy and to explore the correlation of EEG features and clinical Ketogenic diet efficacy. EEG Patients and methods: We retrospectively studied 39 patients with West syndrome who accepted KD therapy from Hypsarrhythmia May 2011 to October 2017. Outcomes including clinical efficacy and EEG features with hypsarrhythmia severity scores were analyzed. Results: After 3 months of treatment, 20 patients (51.3%) had ≥50% seizure reduction, including 4 patients (10.3%) who became seizure-free. After 6 months of treatment, 4 patients remained seizure free, 12 (30.8%) had 90–99% seizure reduction, 8 (20.5%) had a reduction of 50–89%, and 15 (38.5%) had < 50% reduction. Hypsarrhythmia scores were significantly decreased at 3 months of KD. They were associated with seizure outcomes at 6 months independent of gender, the course of disease and etiologies. Patients with a hypsar- rhythmia score ≥8 at 3 months of therapy may not be benefited from KD. Conclusion: Our findings suggest a potential benefit of KD for patients with drug-resistant West syndrome. Early change of EEG after KD may be a predictor of a patient’s response to the therapy. -

Pediatric Disorders
Neurological Disorder Part 3 - Pediatric Disorders CDKL5 Disorder • Characteristics: • Rare x-linked genetic disorder • CDLK5 mutations cause deficiencies in the protein needed for normal brain development • More common in females; however males with the disorder are affected much more severely than females © Trusted Neurodiagnostics Academy CDKL5 Disorder • Characteristics • CDKL5D mutations can be found in children who have been diagnosed with infantile spasms, Lennox Gastaut syndrome, Rett Syndrome, West Syndrome and autism © Trusted Neurodiagnostics Academy CDKL5 Disorder • Symptoms: • Infantile spasms beginning the first 3 - 6 months of life • Neurodevelopmental impairment • Patients cannot walk, talk, or feed themselves • Repetitive hand movements (stereotypies) © Trusted Neurodiagnostics Academy CDKL5 Disorder • Seizures: • Early onset • Infantile spasms, myoclonic, tonic, tonic-clonic seizures • Status epilepticus and non convulsive status epilepticus can occur © Trusted Neurodiagnostics Academy CDKL5 Disorder • Diagnosis: • Genetic blood testing to confirm the change or mutation on the CDKL5 gene • EEG © Trusted Neurodiagnostics Academy CDKL5 Disorder • EEG Findings: • Early in the disorder • EEG may be normal or slightly abnormal • During progression of the disorder • Some background activity is slow and epileptic discharges can be seen in one or more areas • Burst Suppression • Atypical hypsarrhythmia © Trusted Neurodiagnostics Academy CDKL5 Disorder © Trusted Neurodiagnostics Academy CDKL5 Disorder • Treatment: • Seizures -

Levetiracetam-Associated Psychogenic Non-Epileptic Seizures
Original Research DOI: 10.22374/1710-6222.25.2.1 LEVETIRACETAM-ASSOCIATED PSYCHOGENIC NON-EPILEPTIC SEIZURES: A HIDDEN PARADOX Shaik Afshan Jabeen,1 Padmaja Gaddamanugu,1 Ajith Cherian,2 Kandadai Rukmini Mridula,2 Dasari Uday Kumar,1 Angamuttu kanikannan Meena1 1Deptartment of Neurology Nizam’s Institute of Medical Sciences, Hyderabad, India. 2Department of Neurology, Sree Chitra Tirunal Institute of Medical Sciences. Thiruvananthapuram, India. Corresponding Author: [email protected] Submitted: August 16, 2017. Accepted: May 31, 2018. Published: June 15, 2018. ABSTRACT Objectives To study the clinical profile and outcome in patients with epilepsy who developed psychogenic non-epileptic seizures (PNES) associated with levetiracetam (LEV) use. Methods In this prospective observational study, conducted over 1 year, 13 patients with epilepsy and PNES, docu- mented by video electroencephalogram (VEEG) while on LEV, were included. Those with past history of psychiatric illnesses were excluded. VEEG, high-resolution magnetic resonance imaging, neuropsychologi- cal and psychiatric evaluation were performed. Patients in Group I (07) were treated with psychotherapy, psychiatric medications and immediate withdrawal of LEV while, those in Group II (06) received psy- chotherapy, anxiolytics and LEV for initial 2 months after which it was stopped. Follow-up period was six months. Results Mean (±SD) age of patients was 25 ± 12.28 years; there were 11 (84.62%) females. All were on antiepileptic agents which included LEV >1000 mg/day, except one. Mean dose of LEV was 1269.23 ± 483.71 mg/day. J Popul Ther Clin Pharmacol Vol 25(2):1-11; June 15, 2018. This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International License. -
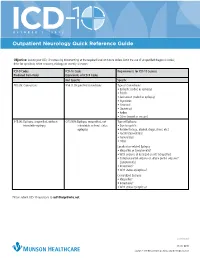
Outpatient Neurology Quick Reference Guide
OCTOBER 1, 2015 Outpatient Neurology Quick Reference Guide Objective: Ensure your ICD-10 success by documenting at the required level on future orders. Limit the use of unspecified diagnosis codes; drive for specificity when anatomy, etiology, or severity is known. ICD-9 Codes ICD-10 Code Requirements for ICD-10 Success Produced from Order Equivalents of ICD-9 Codes Not Specific Specific 780.39: Convulsions R56.9: Unspecified convulsions Type of Convulsions: • Epileptic (coded as epilepsy) • Febrile • Jacksonian (coded as epilepsy) • Myoclonic • Neonatal • Obstetrical • Reflex • Other (coded as seizure) 345.90: Epilepsy, unspecified, without G40.909: Epilepsy, unspecified, not Type of Epilepsy: intractable epilepsy intractable, without status • Due to syphilis epileptus • Related to (e.g., alcohol, drugs, stress, etc.) • Localization-related • Generalized • Other Localization-related Epilepsy • Idiopathic or Symptomatic? • With seizures of localized onset? (idiopathic) • Complex partial seizures or simple partial seizures? (symptomatic) • Intractable? • With status epilepticus? Generalized Epilepsy • Idiopathic? • Intractable? • With status epilepticus? Please submit ICD-10 questions to [email protected]. continued 11372 08/15 Copyright © 2015 Munson Healthcare, Traverse City, MI. All rights reserved. OCTOBER 1, 2015 Outpatient Neurology Quick Reference Guide Specific ICD-10-CM Codes Code Definition Convulsions R56.01 Complex febrile convulsions R53.00 Simple febrile convulsions G25.3 Myoclonus R25.8 Other abnormal involuntary movements -

Investigation of Seizures in Infants
Chapter 23 Investigation of seizures in infants RICHARD E. APPLETON1 and AILSA McLELLAN2 1The Roald Dahl EEG Unit, Paediatric Neurosciences Foundation, Alder Hey Children’s NHS Foundation Trust, Liverpool, and 2Department of Paediatric Neurosciences, Royal Hospital for Sick Children, Edinburgh The investigation of seizures in infancy (i.e. within the first year of life) begins with establishing whether the seizures are epileptic or non-epileptic in origin. The ‘broad’ differential diagnosis of possible seizures and ‘epilepsy’ is multiple and is particularly difficult under the age of 12 months and includes: Gastro-oesophageal reflux (Sandifer’s syndrome) Pallid syncopal attacks (reflex anoxic seizures) Cyanotic breath-holding attacks Cardiac arrhythmias Münchausen syndrome by proxy (passive or active both representing a form of child abuse) Shuddering spells and jitteriness Hyperekplexia Benign neonatal sleep myoclonus Benign myoclonus of infancy Tonic reflex activity and involuntary movements (seen in children with neurological impairment including cerebral palsy or hydrocephalus). Once a non-epileptic disorder has been excluded or the episodes are considered to be obviously epileptic, then the following conditions/investigations should be considered on a chronological basis. Perinatal (first week of life) and neonatal (first month of life) seizures The newborn period is the time of life with the highest risk of seizures1-3. This is because of the relative lack, and immature development of inhibitory neurotransmitters and their pathways. The immature and developing brain is susceptible to a large number of both cerebral and systemic insults including: Asphyxia (hypoxic-ischaemic encephalopathy) the most common and also most serious cause of neonatal seizures – particularly in term infants Intra- and periventricular haemorrhage – particularly in pre-term infants Metabolic dysfunction (e.g. -

Genes of Early-Onset Epileptic Encephalopathies: from Genotype to Phenotype
Pediatric Neurology 46 (2012) 24e31 Contents lists available at ScienceDirect Pediatric Neurology journal homepage: www.elsevier.com/locate/pnu Review Article Genes of Early-Onset Epileptic Encephalopathies: From Genotype to Phenotype Mario Mastrangelo MD, Vincenzo Leuzzi MD * Division of Child Neurology, Department of Pediatrics, Child Neurology, and Psychiatry, Sapienza University of Rome, Rome, Italy article information abstract Article history: Early-onset epileptic encephalopathies are severe disorders in which cognitive, sensory, and motor Received 26 July 2011 development is impaired by recurrent clinical seizures or prominent interictal epileptiform discharges Accepted 24 October 2011 during the neonatal or early infantile periods. They include Ohtahara syndrome, early myoclonic epileptic encephalopathy, West syndrome, Dravet syndrome, and other diseases, e.g., X-linked myoclonic seizures, spasticity and intellectual disability syndrome, idiopathic infantile epileptic-dyskinetic encephalopathy, epilepsy and mental retardation limited to females, and severe infantile multifocal epilepsy. We summarize recent updates on the genes and related clinical syndromes involved in the pathogenesis of early-onset epileptic encephalopathies: Aristaless-related homeobox (ARX), cyclin- dependent kinase-like 5 (CDKL5), syntaxin-binding protein 1 (STXBP1), solute carrier family 25 member 22 (SLC25A22), nonerythrocytic a-spectrin-1 (SPTAN1), phospholipase Cb1(PLCb1), membrane- associated guanylate kinase inverted-2 (MAGI2), polynucleotide kinase -

Infantile Spasms: an Update on Pre-Clinical Models and EEG Mechanisms
children Review Infantile Spasms: An Update on Pre-Clinical Models and EEG Mechanisms Remi Janicot, Li-Rong Shao and Carl E. Stafstrom * Division of Pediatric Neurology, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA; [email protected] (R.J.); [email protected] (L.-R.S.) * Correspondence: [email protected]; Tel.: +1-(410)-955-4259; Fax: +1-(410)-614-2297 Received: 19 November 2019; Accepted: 23 December 2019; Published: 6 January 2020 Abstract: Infantile spasms (IS) is an epileptic encephalopathy with unique clinical and electrographic features, which affects children in the middle of the first year of life. The pathophysiology of IS remains incompletely understood, despite the heterogeneity of IS etiologies, more than 200 of which are known. In particular, the neurobiological basis of why multiple etiologies converge to a relatively similar clinical presentation has defied explanation. Treatment options for this form of epilepsy, which has been described as “catastrophic” because of the poor cognitive, developmental, and epileptic prognosis, are limited and not fully effective. Until the pathophysiology of IS is better clarified, novel treatments will not be forthcoming, and preclinical (animal) models are essential for advancing this knowledge. Here, we review preclinical IS models, update information regarding already existing models, describe some novel models, and discuss exciting new data that promises to advance understanding of the cellular mechanisms underlying the specific EEG changes seen in IS—interictal hypsarrhythmia and ictal electrodecrement. Keywords: infantile spasms; West syndrome; epilepsy; childhood; epileptic encephalopathy; electroencephalogram (EEG); hypsarrhythmia; electrodecrement; animal model 1. Introduction Epileptic encephalopathies (EEs) are a spectrum of disorders that mostly begin during infancy and have poor neurological and behavioral outcomes. -

Epilepsy and Mitochondrial Dysfunction: ª the Author(S) 2017 DOI: 10.1177/2326409817733012 a Single Center’S Experience Journals.Sagepub.Com/Home/Iem
Original Article Journal of Inborn Errors of Metabolism & Screening 2017, Volume 5: 1–12 Epilepsy and Mitochondrial Dysfunction: ª The Author(s) 2017 DOI: 10.1177/2326409817733012 A Single Center’s Experience journals.sagepub.com/home/iem Russell P. Saneto, DO, PhD1 Abstract Epilepsy is a common manifestation of mitochondrial disease. In a large cohort of children and adolescents with mitochondrial disease (n ¼ 180), over 48% of patients developed seizures. The majority (68%) of patients were younger than 3 years and medically intractable (90%). The electroencephalographic pattern of multiregional epileptiform discharges over the left and right hemisphere with background slowing occurred in 62%. The epilepsy syndrome, infantile spasms, was seen in 17%. Polymerase g mutations were the most common genetic etiology of seizures, representing Alpers-Huttenlocher syndrome (14%). The severity of disease in those patients with epilepsy was significant, as 13% of patients experienced early death. Simply the loss of energy production cannot explain the development of seizures or all patients with mitochondrial dysfunction would have epilepsy. Until the various aspects of mitochondrial physiology that are involved in proper brain development are understood, epilepsy and its treatment will remain unsatisfactory. Keywords epilepsy, seizures, mitochondrial disease, electroencephalogram, infantile spasms, Alpers-Huttenlocher syndrome, status epilep- ticus, treatment Introduction wide variety of clinical phenotypes, including the high preva- lence of seizures and encephalopathy in mitochondrial diseases. Mitochondria are essential organelles involved in the proper Several studies have shown that approximately 35% to 60% of operation of the highly controlled cellular energetic processes individuals with biochemically confirmed mitochondrial disease of brain function, including amino acid and fatty acid synthesis 3–7 have epilepsy. -

Epileptic Spasms in Clusters Without Hypsarrhythmia in Infancy
Original article Epileptic Disord 2003; 5: 109-13 Epileptic spasms in clusters without hypsarrhythmia in infancy Roberto Horacio Caraballo1, Natalio Fejerman1, Bernardo Dalla Bernardina2, Victor Ruggieri1, Ricardo Cersósimo1, Carlos Medina3, Juan Pociecha3 1. Servicio de Neurología, Hospital de Niños “Prof. Dr. Juan P. Garrahan”, Buenos Aires, Argentina. 2. Neuropediatric Department, Borgo Roma Hospital, University of Verona, Italy. 3. Servicio de Neurofisiología, Hospital de Niños “Prof. Dr. Juan P. Garrahan”, Buenos Aires, Argentina. Received December 31, 2002; Accepted April 25, 2003 ABSTRACT − Spasms are defined as epileptic seizures characterized by brief axial contraction, in flexion, extension or mixed, symmetric or asymmetric, lasting from a fraction of a second to 1-2s, and are associated with a slow-wave transient or sharp and slow-wave complex, followed or not by voltage attenu- ation. Epileptic spasms usually appear in clusters and are age-dependent. This type of epileptic spasms associated with the particular EEG pattern, hypsar- rhythmia, constitutes the basis for the diagnosis of West syndrome. The question is, how to nosologically define those patients who clearly present epileptic spasms in clusters without modified or typical hypsarrhythmia and with or without focal paroxysmal discharges on the interictal EEG. In the present series, the four patients show that epileptic spasms in clusters may occur in infancy, without hypsarrhythmia. They all presented the following features: normal neuropsychological development before onset of epileptic spasms, clusters of epileptic spasms, focal clinical and/or EEG abnormalities, normal neuroradiological imaging, neurometabolic investigations and karyotypes. In three of the patients, seizures were refractory to AEDs. Epileptic spasms in clusters without hypsarrhythmia that start in the first year of life represent a subtype of infantile spasms that generally are refractory to AEDs. -

Temporal Lobe Epilepsy Semiology
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 751510, 10 pages doi:10.1155/2012/751510 Review Article Temporal Lobe Epilepsy Semiology Robert D. G. Blair Division of Neurology, Department of Medicine, Credit Valley Hospital, University of Toronto, Mississauga, ON, Canada L5M 2N1 Correspondence should be addressed to Robert D. G. Blair, [email protected] Received 22 October 2011; Accepted 26 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 Robert D. G. Blair. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Epilepsy represents a multifaceted group of disorders divided into two broad categories, partial and generalized, based on the seizure onset zone. The identification of the neuroanatomic site of seizure onset depends on delineation of seizure semiology by a careful history together with video-EEG, and a variety of neuroimaging technologies such as MRI, fMRI, FDG-PET, MEG, or invasive intracranial EEG recording. Temporal lobe epilepsy (TLE) is the commonest form of focal epilepsy and represents almost 2/3 of cases of intractable epilepsy managed surgically. A history of febrile seizures (especially complex febrile seizures) is common in TLE and is frequently associated with mesial temporal sclerosis (the commonest form of TLE). Seizure auras occur in many TLE patients and often exhibit features that are relatively specific for TLE but few are of lateralizing value. Automatisms, however, often have lateralizing significance. Careful study of seizure semiology remains invaluable in addressing the search for the seizure onset zone. -

Pediatric Epilepsy
PEDIATRIC EPILEPSY Ø Epilepsy is one of the most common chronic neurological disorders. It is characterized by recurrent unprovoked seizures or an enduring predisposition to generate epileptic seizures. If epilepsy begins in childhood, it is often outgrown. Seizures are common in childhood and adolescence. Approximately 3% of children will experience a seizure. Ø A seizure occurs when there is a sudden change in behavior or sensation caused by abnormal and excessive electrical hypersynchronization of neuronal networks in the cerebral cortex. Normal inhibition is overcome by excessive excitatory stimuli. Ø If the cause of the seizures is known (for example: genetic, inborn errors of metabolism, metabolic (eg: low glucose, electrolyte abnormalities), structural (eg: malformations, tumours, bleeds, stroke, traumatic brain injury), infectious, inflammatory, or toxins) it is classified as symptomatic. If the cause is unknown, it is classified as idiopathic. 1. WHERE DID THE SEIZURE START? / WHAT KIND OF SEIZURE IS IT? 2. IS AWARENESS YES FOCAL ONSET GENERALIZED UNKNOWN IMPAIRED? NO Seizure that originates ONSET ONSET in a focal cortical area Seizure that involves When it is unclear YES with associated clinical both sides of the where the seizure 3. PROGRESSION TO BILATERAL? features. brain from the onset. starts. NO SEIZURE SEMIOLOGY (The terminology for seizure types is designed to be useful for communicating the key characteristics of seizures) CLONIC: sustained rhythmical TONIC: muscles stiffen or ATONIC: sudden loss of muscle tone, MYOCLONUS: sudden lighting- jerking movements. tense. lasting seconds. like jerk, may cluster. EPILEPTIC SPASM: sudden AUTONOMIC: eg: AUTOMATISMS: ABSENCE: brief (≤ 10s), OTHERS: change flexion, extension, or flexion- rising epigastric stereotyped, purposeless frequent (up to 100’s) in cognition, extension of proximal and sensations, waves of movements.