Genes of Early-Onset Epileptic Encephalopathies: from Genotype to Phenotype
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Childhood Epilepsy: an Update on Diagnosis and Management
American Journal of Neuroscience Review Childhood Epilepsy: An Update on Diagnosis and Management Khaled Saad Department of Pediatrics, Faculty of Medicine, University of Assiut, Assiut 71516, Egypt Article history Abstract: Within the past few years there is a rapid expansion in our Received: 23-10-2014 understanding of epilepsy. The development of new anti-epileptic drugs and Revised: 25-11-2014 refinements to epilepsy surgery are widening the therapeutic options for Accepted: 12-01-2015 epilepsy. In addition, the classification of the epilepsies continues to evolve based on an increased understanding of the molecular genetics of the condition and this includes the recognition of possible novel epilepsy syndromes. This review considers some of these exciting developments, as well as addressing the essential features of the diagnosis, investigations, management and impact of epilepsy in childhood. Keywords: Children, Epilepsy, Seizure, Anti-Epileptic Drugs Introduction guidelines for optimal practices, rational therapy and counseling (Aylwad, 2008; Tamber and Mountz, 2012; Epilepsy is a common heterogeneous neurological Sharma, 2013). problem in children. It exerts a significant physical, psychological, economic and social toll on children and Definitions their caregivers. Fifty million people have epilepsies A seizure is defined as an excessive burst of abnormal globally; more than half of them are children. In the synchronized neuronal activity affecting small or large USA; between 25,000 and 40,000 children will have a neuronal networks that results in clinical manifestations first non-febrile seizure each year. The problem is that are sudden, transient and usually brief. Epilepsy is further compounded in developing countries as they add defined as a disorder of the brain characterized by any of about 75-80% of new cases of epilepsy (Guerrini, 2006; the following conditions: (1) At least two unprovoked (or Tamber and Mountz, 2012; Sharma, 2013). -

Causes of Mortality in Early Infantile Epileptic Encephalopathy: a Systematic Review
Epilepsy & Behavior 85 (2018) 32–36 Contents lists available at ScienceDirect Epilepsy & Behavior journal homepage: www.elsevier.com/locate/yebeh Review Causes of mortality in early infantile epileptic encephalopathy: A systematic review Graciane Radaelli a,b, Francisco de Souza Santos b, Wyllians Vendramini Borelli b,LeonardoPisanib, Magda Lahorgue Nunes b,d, Fulvio Alexandre Scorza c,d, Jaderson Costa da Costa b,d,⁎ a Federal University of São Paulo (UNIFESP)/Paulista School of Medicine, São Paulo, Brazil b Brain Institute of Rio Grande do Sul (BraIns), Pontifical Catholic University of Rio Grande do Sul, Porto Alegre, RS, Brazil c Laboratory of Neuroscience, Department of Neurology and Neurosurgery, Federal University of São Paulo, São Paulo, SP, Brazil d CNPq, Brazil article info abstract Article history: Introduction: Early infantile epileptic encephalopathy syndrome (EIEE), also known as Ohtahara syndrome, is an Received 6 March 2018 age-dependent epileptic encephalopathy syndrome defined by clinical features and electroencephalographic Revised 25 April 2018 findings. Epileptic disorders with refractory seizures beginning in the neonatal period and/or early infancy Accepted 5 May 2018 have a potential risk of premature mortality, including sudden death. We aimed to identify the causes of death Available online xxxx in EIEE and conducted a literature survey of fatal outcomes. Methods: We performed a literature search in MEDLINE, EMBASE, and Web of Science for data from inception Keywords: “ ”“ ”“ ”“ ” “ ” Ohtahara syndrome until September 2017. The terms death sudden, unexplained death, SUDEP, lethal, and fatal and the “ ”“ ”“ ”“ Early infantile epileptic syndrome medical subject heading terms epileptic encephalopathy, mortality, death, sudden infant death syn- Suppression burst drome,” and “human” were used in the search strategy. -

Epilepsy in Childhood
Psychogenic Non-epileptic Seizures Dr Katharina Junejo Consultant Child and Adolescent Psychiatrist Aim Overview of basic principles in childhood epilepsy PNES Diagnosis of epilepsy Every child’s brain is capable of generating seizures with certain pro-convulsive drugs, acute metabolic disturbance, CNS infection, acute head trauma Risk of epilepsy after acute and provoked seizure is 3-5% Emotional stress is not considered provoking factor Henri Gastaut and colleagues proposed a classification of seizures: “all attempts at classification of seizures are hampered by our limited knowledge of the underlying pathological processes within the brain and that any classification must of necessity be a tentative one and will be subject to change with every advance in scientific understanding of epilepsy” (Gastaut et al 1964). Epilepsy has variable clinical presentations Convulsions (tonic- Staring spells clonic seizures) (absences) Period with confusion with or without Variants of automatism (complex paroxysmal events partial seizures) during sleep (common frontal lobe seizures) Limb or body jerk movements (myoclonus) Loss of speech/decline in Spasms (infantile or social, cognitive function Falls or drops epileptic spasms) (Landau-Kleffner (atonic but also syndrome) tonic seizures) ILAE Revised Terminology for Organization of Seizures and Epilepsies 2011 ‐ 2013 Classification of seizures: generalised and focal seizures Electroclinical syndromes and other epilepsies grouped by specificity of diagnosis (arranged by typical age of onset) Major conceptual changes: using aetiological classification of epilepsy-genetic, metabolic, immune, infectious and unknown causes Changes in terminology Operational diagnosis of epilepsy: occurrence of 2 or more unprovoked seizures, irrespective of seizure type (as recurrence risk is 80-90%) Careful history is only diagnostic test as many paroxysmal disorders that mimic epileptic seizures (most common vasovagal syncope or reflex anoxic seizure) Limitations of EEG: approx. -

Prognostic Utility of Hypsarrhythmia Scoring in Children with West
Clinical Neurology and Neurosurgery 184 (2019) 105402 Contents lists available at ScienceDirect Clinical Neurology and Neurosurgery journal homepage: www.elsevier.com/locate/clineuro Prognostic utility of hypsarrhythmia scoring in children with West syndrome T after ketogenic diet ⁎ Yunjian Zhang1, Lifei Yu1, Yuanfeng Zhou, Linmei Zhang, Yi Wang, Shuizhen Zhou Department of Pediatric Neurology, Children’s Hospital of Fudan University, China ARTICLE INFO ABSTRACT Keywords: Objective: The aim of this study was to evaluate the clinical efficacy and electroencephalographic (EEG) changes West syndrome of West syndrome after ketogenic diet (KD) therapy and to explore the correlation of EEG features and clinical Ketogenic diet efficacy. EEG Patients and methods: We retrospectively studied 39 patients with West syndrome who accepted KD therapy from Hypsarrhythmia May 2011 to October 2017. Outcomes including clinical efficacy and EEG features with hypsarrhythmia severity scores were analyzed. Results: After 3 months of treatment, 20 patients (51.3%) had ≥50% seizure reduction, including 4 patients (10.3%) who became seizure-free. After 6 months of treatment, 4 patients remained seizure free, 12 (30.8%) had 90–99% seizure reduction, 8 (20.5%) had a reduction of 50–89%, and 15 (38.5%) had < 50% reduction. Hypsarrhythmia scores were significantly decreased at 3 months of KD. They were associated with seizure outcomes at 6 months independent of gender, the course of disease and etiologies. Patients with a hypsar- rhythmia score ≥8 at 3 months of therapy may not be benefited from KD. Conclusion: Our findings suggest a potential benefit of KD for patients with drug-resistant West syndrome. Early change of EEG after KD may be a predictor of a patient’s response to the therapy. -

Neonatal Seizures Revisited
children Review Neonatal Seizures Revisited Konrad Kaminiów 1 , Sylwia Kozak 1 and Justyna Paprocka 2,* 1 Students’ Scientific Society, Department of Pediatric Neurology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, 40-752 Katowice, Poland; [email protected] (K.K.); [email protected] (S.K.) 2 Department of Pediatric Neurology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, 40-752 Katowice, Poland * Correspondence: [email protected] Abstract: Seizures are the most common neurological disorder in newborns and are most prevalent in the neonatal period. They are mostly caused by severe disorders of the central nervous system (CNS). However, they can also be a sign of the immaturity of the infant’s brain, which is characterized by the presence of specific factors that increase excitation and reduce inhibition. The most common disorders which result in acute brain damage and can manifest as seizures in neonates include hypoxic-ischemic encephalopathy (HIE), ischemic stroke, intracranial hemorrhage, infections of the CNS as well as electrolyte and biochemical disturbances. The therapeutic management of neonates and the prognosis are different depending on the etiology of the disorders that cause seizures which can lead to death or disability. Therefore, establishing a prompt diagnosis and implementing appropriate treatment are significant, as they can limit adverse long-term effects and improve outcomes. In this review paper, we present the latest reports on the etiology, pathomechanism, clinical symptoms and guidelines for the management of neonates with acute symptomatic seizures. Keywords: neonatal seizures; pathophysiology; genetics; inborn errors of metabolism Citation: Kaminiów, K.; Kozak, S.; Paprocka, J. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub
US 2010O2.10567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub. Date: Aug. 19, 2010 (54) USE OF ATUFTSINASATHERAPEUTIC Publication Classification AGENT (51) Int. Cl. A638/07 (2006.01) (76) Inventor: Dorian Bevec, Germering (DE) C07K 5/103 (2006.01) A6IP35/00 (2006.01) Correspondence Address: A6IPL/I6 (2006.01) WINSTEAD PC A6IP3L/20 (2006.01) i. 2O1 US (52) U.S. Cl. ........................................... 514/18: 530/330 9 (US) (57) ABSTRACT (21) Appl. No.: 12/677,311 The present invention is directed to the use of the peptide compound Thr-Lys-Pro-Arg-OH as a therapeutic agent for (22) PCT Filed: Sep. 9, 2008 the prophylaxis and/or treatment of cancer, autoimmune dis eases, fibrotic diseases, inflammatory diseases, neurodegen (86). PCT No.: PCT/EP2008/007470 erative diseases, infectious diseases, lung diseases, heart and vascular diseases and metabolic diseases. Moreover the S371 (c)(1), present invention relates to pharmaceutical compositions (2), (4) Date: Mar. 10, 2010 preferably inform of a lyophilisate or liquid buffersolution or artificial mother milk formulation or mother milk substitute (30) Foreign Application Priority Data containing the peptide Thr-Lys-Pro-Arg-OH optionally together with at least one pharmaceutically acceptable car Sep. 11, 2007 (EP) .................................. O7017754.8 rier, cryoprotectant, lyoprotectant, excipient and/or diluent. US 2010/0210567 A1 Aug. 19, 2010 USE OF ATUFTSNASATHERAPEUTIC ment of Hepatitis BVirus infection, diseases caused by Hepa AGENT titis B Virus infection, acute hepatitis, chronic hepatitis, full minant liver failure, liver cirrhosis, cancer associated with Hepatitis B Virus infection. 0001. The present invention is directed to the use of the Cancer, Tumors, Proliferative Diseases, Malignancies and peptide compound Thr-Lys-Pro-Arg-OH (Tuftsin) as a thera their Metastases peutic agent for the prophylaxis and/or treatment of cancer, 0008. -

Pediatric Disorders
Neurological Disorder Part 3 - Pediatric Disorders CDKL5 Disorder • Characteristics: • Rare x-linked genetic disorder • CDLK5 mutations cause deficiencies in the protein needed for normal brain development • More common in females; however males with the disorder are affected much more severely than females © Trusted Neurodiagnostics Academy CDKL5 Disorder • Characteristics • CDKL5D mutations can be found in children who have been diagnosed with infantile spasms, Lennox Gastaut syndrome, Rett Syndrome, West Syndrome and autism © Trusted Neurodiagnostics Academy CDKL5 Disorder • Symptoms: • Infantile spasms beginning the first 3 - 6 months of life • Neurodevelopmental impairment • Patients cannot walk, talk, or feed themselves • Repetitive hand movements (stereotypies) © Trusted Neurodiagnostics Academy CDKL5 Disorder • Seizures: • Early onset • Infantile spasms, myoclonic, tonic, tonic-clonic seizures • Status epilepticus and non convulsive status epilepticus can occur © Trusted Neurodiagnostics Academy CDKL5 Disorder • Diagnosis: • Genetic blood testing to confirm the change or mutation on the CDKL5 gene • EEG © Trusted Neurodiagnostics Academy CDKL5 Disorder • EEG Findings: • Early in the disorder • EEG may be normal or slightly abnormal • During progression of the disorder • Some background activity is slow and epileptic discharges can be seen in one or more areas • Burst Suppression • Atypical hypsarrhythmia © Trusted Neurodiagnostics Academy CDKL5 Disorder © Trusted Neurodiagnostics Academy CDKL5 Disorder • Treatment: • Seizures -

Levetiracetam-Associated Psychogenic Non-Epileptic Seizures
Original Research DOI: 10.22374/1710-6222.25.2.1 LEVETIRACETAM-ASSOCIATED PSYCHOGENIC NON-EPILEPTIC SEIZURES: A HIDDEN PARADOX Shaik Afshan Jabeen,1 Padmaja Gaddamanugu,1 Ajith Cherian,2 Kandadai Rukmini Mridula,2 Dasari Uday Kumar,1 Angamuttu kanikannan Meena1 1Deptartment of Neurology Nizam’s Institute of Medical Sciences, Hyderabad, India. 2Department of Neurology, Sree Chitra Tirunal Institute of Medical Sciences. Thiruvananthapuram, India. Corresponding Author: [email protected] Submitted: August 16, 2017. Accepted: May 31, 2018. Published: June 15, 2018. ABSTRACT Objectives To study the clinical profile and outcome in patients with epilepsy who developed psychogenic non-epileptic seizures (PNES) associated with levetiracetam (LEV) use. Methods In this prospective observational study, conducted over 1 year, 13 patients with epilepsy and PNES, docu- mented by video electroencephalogram (VEEG) while on LEV, were included. Those with past history of psychiatric illnesses were excluded. VEEG, high-resolution magnetic resonance imaging, neuropsychologi- cal and psychiatric evaluation were performed. Patients in Group I (07) were treated with psychotherapy, psychiatric medications and immediate withdrawal of LEV while, those in Group II (06) received psy- chotherapy, anxiolytics and LEV for initial 2 months after which it was stopped. Follow-up period was six months. Results Mean (±SD) age of patients was 25 ± 12.28 years; there were 11 (84.62%) females. All were on antiepileptic agents which included LEV >1000 mg/day, except one. Mean dose of LEV was 1269.23 ± 483.71 mg/day. J Popul Ther Clin Pharmacol Vol 25(2):1-11; June 15, 2018. This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International License. -

Genetic and Phenotypic Heterogeneity Suggest Therapeutic Implications in SCN2A-Related Disorders
doi:10.1093/brain/awx054 BRAIN 2017: Page 1 of 21 | 1 Genetic and phenotypic heterogeneity suggest therapeutic implications in SCN2A-related disorders Markus Wolff,1,Ã Katrine M. Johannesen,2,3,Ã Ulrike B. S. Hedrich,4,Ã Silvia Masnada,5 Guido Rubboli,2,6 Elena Gardella,2,3 Gaetan Lesca,7,8,9 Dorothe´e Ville,10 Mathieu Milh,11,12 Laurent Villard,12 Alexandra Afenjar,13 Sandra Chantot-Bastaraud,13 Cyril Mignot,14 Caroline Lardennois,15 Caroline Nava,16,17 Niklas Schwarz,4 Marion Ge´rard,18 Laurence Perrin,19 Diane Doummar,20 Ste´phane Auvin,21,22 Maria J. Miranda,23 Maja Hempel,24 Eva Brilstra,25 Nine Knoers,25 Nienke Verbeek,25 Marjan van Kempen,25 Kees P. Braun,26 Grazia Mancini,27 Saskia Biskup,28 Konstanze Ho¨rtnagel,28 Miriam Do¨cker,28 Thomas Bast,29 Tobias Loddenkemper,30 Lily Wong-Kisiel,31 Friedrich M. Baumeister,32 Walid Fazeli,33 Pasquale Striano,34 Robertino Dilena,35 Elena Fontana,36 Federico Zara,37 Gerhard Kurlemann,38 Joerg Klepper,39 Jess G. Thoene,40 Daniel H. Arndt,41 Nicolas Deconinck,42 Thomas Schmitt-Mechelke,43 Oliver Maier,44 Hiltrud Muhle,45 Beverly Wical,46 Claudio Finetti,47 Reinhard Bru¨ckner,48 Joachim Pietz,49 Gu¨nther Golla,50 Dinesh Jillella,51 Karen M. Linnet,52 Perrine Charles,53 Ute Moog,54 Eve O˜ iglane-Shlik,55 John F. Mantovani,56 Kristen Park,57 Marie Deprez,58 Damien Lederer,58 Sandrine Mary,58 Emmanuel Scalais,59 Laila Selim,60 Rudy Van Coster,61 Lieven Lagae,62 Marina Nikanorova,2 Helle Hjalgrim,2,3 G. -

Childhood Neurological Conditions and Disorders
Childhood neurological conditions and disorders Please note: We do include rare conditions. If your one is not in the list below we will still consider your survey responses. Epilepsies Autosomal dominant epilepsy with auditory features (ADEAF) Autosomal-dominant nocturnal frontal lobe epilepsy (ADNFLE) Benign epilepsy with centrotemporal spikes (BECTS) Benign familial infantile epilepsy Benign familial neonatal epilepsy (BFNE) Benign infantile epilepsy Benign neonatal seizures (BNS) Childhood absence epilepsy (CAE) Cyclin Dependent Kinase-Like 5 (CDKL5) Deficiency Disorder Dravet syndrome Early myoclonic encephalopathy (EME) Epilepsy of infancy with migrating focal seizures Epilepsy with generalized tonic–clonic seizures alone Epilepsy with myoclonic absences Epilepsy with myoclonic atonic (previously astatic) seizures Epileptic encephalopathy with continuous spike-and-wave during sleep (CSWS) Familial focal epilepsy with variable foci (childhood to adult) Febrile seizures (FS) Febrile seizures plus (FS+) (can start in infancy) Gelastic seizures with hypothalamic hamartoma Hemiconvulsion–hemiplegia–epilepsy Juvenile absence epilepsy (JAE) Juvenile myoclonic epilepsy (JME) Landau-Kleffner syndrome (LKS) Late onset childhood occipital epilepsy (Gastaut type) Lennox-Gastaut syndrome Mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE with HS) Myoclonic epilepsy in infancy (MEI) Ohtahara syndrome Panayiotopoulos syndrome Photosensitive absence epilepsies including Jeavons syndrome, Sunflower syndrome Progressive myoclonus epilepsies -
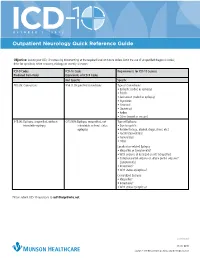
Outpatient Neurology Quick Reference Guide
OCTOBER 1, 2015 Outpatient Neurology Quick Reference Guide Objective: Ensure your ICD-10 success by documenting at the required level on future orders. Limit the use of unspecified diagnosis codes; drive for specificity when anatomy, etiology, or severity is known. ICD-9 Codes ICD-10 Code Requirements for ICD-10 Success Produced from Order Equivalents of ICD-9 Codes Not Specific Specific 780.39: Convulsions R56.9: Unspecified convulsions Type of Convulsions: • Epileptic (coded as epilepsy) • Febrile • Jacksonian (coded as epilepsy) • Myoclonic • Neonatal • Obstetrical • Reflex • Other (coded as seizure) 345.90: Epilepsy, unspecified, without G40.909: Epilepsy, unspecified, not Type of Epilepsy: intractable epilepsy intractable, without status • Due to syphilis epileptus • Related to (e.g., alcohol, drugs, stress, etc.) • Localization-related • Generalized • Other Localization-related Epilepsy • Idiopathic or Symptomatic? • With seizures of localized onset? (idiopathic) • Complex partial seizures or simple partial seizures? (symptomatic) • Intractable? • With status epilepticus? Generalized Epilepsy • Idiopathic? • Intractable? • With status epilepticus? Please submit ICD-10 questions to [email protected]. continued 11372 08/15 Copyright © 2015 Munson Healthcare, Traverse City, MI. All rights reserved. OCTOBER 1, 2015 Outpatient Neurology Quick Reference Guide Specific ICD-10-CM Codes Code Definition Convulsions R56.01 Complex febrile convulsions R53.00 Simple febrile convulsions G25.3 Myoclonus R25.8 Other abnormal involuntary movements -

PAEDIATRIC PROTOCOLS for Malaysian Hospitals 3Rd Edition
PAEDIATRIC PROTOCOLS For Malaysian Hospitals 3rd Edition Hussain Imam Hj Muhammad Ismail Ng Hoong Phak Terrence Thomas Kementerian Kesihatan Malaysia PAEDIATRIC PROTOCOLS For Malaysian Hospitals 3rd Edition Hussain Imam Hj Muhammad Ismail Ng Hoong Phak Terrence Thomas Scan this QR code to download the electronic Paediatric Protocol 3rd Edition Kementerian Kesihatan Malaysia i ii FOREWORD BY THE DIRECTOR GENERAL OF HEALTH Malaysia like the rest of the world has 3 more years to achieve the Millennium Developmental Goals (MDG). MDG 4 is concerned with under 5 mortality. Although we have done very well since lndependence to reduce our infant and toddler mortality rates, we are now faced with some last lap issues in achieving this goal. Despite urbanization there are still many children in the rural areas. This constitutes a vulnerable group in many ways. Among the factors contributing to this vulner- ability is the distance from specialist care. There is a need to ensure that doctors in the frontline are well equipped to handle common paediatric emergencies so that proper care can be instituted from the very beginning. Although all doctors are now required to do 4 months of pre-registration training in Paediatrics, this is insufficient to prepare them for all the conditions they are likely to meet as Medical Officers in district hospitals and health clinics. Hence the effort made by the paediatricians to prepare a protocol book covering all the common paediatric problems is laudable. I would also like to congratulate them for bringing out a third edition within 4 years of the previous edition. l am confident that this third edition will contribute to improving the care of children attending the Ministry’s facilities throughout the country.