0922 WSCC Final.Pages
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Intervertebral and Epiphyseal Fusion in the Postnatal Ontogeny of Cetaceans and Terrestrial Mammals
J Mammal Evol DOI 10.1007/s10914-014-9256-7 ORIGINAL PAPER Intervertebral and Epiphyseal Fusion in the Postnatal Ontogeny of Cetaceans and Terrestrial Mammals Meghan M. Moran & Sunil Bajpai & J. Craig George & Robert Suydam & Sharon Usip & J. G. M. Thewissen # Springer Science+Business Media New York 2014 Abstract In this paper we studied three related aspects of the Introduction ontogeny of the vertebral centrum of cetaceans and terrestrial mammals in an evolutionary context. We determined patterns The vertebral column provides support for the body and of ontogenetic fusion of the vertebral epiphyses in bowhead allows for flexibility and mobility (Gegenbaur and Bell whale (Balaena mysticetus) and beluga whale 1878;Hristovaetal.2011; Bruggeman et al. 2012). To (Delphinapterus leucas), comparing those to terrestrial mam- achieve this mobility, individual vertebrae articulate with each mals and Eocene cetaceans. We found that epiphyseal fusion other through cartilaginous intervertebral joints between the is initiated in the neck and the sacral region of terrestrial centra and synovial joints between the pre- and post- mammals, while in recent aquatic mammals epiphyseal fusion zygapophyses. The mobility of each vertebral joint varies is initiated in the neck and caudal regions, suggesting loco- greatly between species as well as along the vertebral column motor pattern and environment affect fusion pattern. We also within a single species. Vertebral column mobility greatly studied bony fusion of the sacrum and evaluated criteria used impacts locomotor style, whether the animal is terrestrial or to homologize cetacean vertebrae with the fused sacrum of aquatic. In aquatic Cetacea, buoyancy counteracts gravity, and terrestrial mammals. We found that the initial ossification of the tail is the main propulsive organ (Fish 1996;Fishetal. -

The Epiphyseal Plate: Physiology, Anatomy, and Trauma*
3 CE CREDITS CE Article The Epiphyseal Plate: Physiology, Anatomy, and Trauma* ❯❯ Dirsko J. F. von Pfeil, Abstract: This article reviews the development of long bones, the microanatomy and physiology Dr.med.vet, DVM, DACVS, of the growth plate, the closure times and contribution of different growth plates to overall growth, DECVS and the effect of, and prognosis for, traumatic injuries to the growth plate. Details on surgical Veterinary Specialists of Alaska Anchorage, Alaska treatment of growth plate fractures are beyond the scope of this article. ❯❯ Charles E. DeCamp, DVM, MS, DACVS athologic conditions affecting epi foramen. Growth factors and multipotent Michigan State University physeal (growth) plates in imma stem cells support the formation of neo ture animals may result in severe natal bone consisting of a central marrow P 2 orthopedic problems such as limb short cavity surrounded by a thin periosteum. ening, angular limb deformity, or joint The epiphysis is a secondary ossifica incongruity. Understanding growth plate tion center in the hyaline cartilage forming anatomy and physiology enables practic the joint surfaces at the proximal and distal At a Glance ing veterinarians to provide a prognosis ends of the bones. Secondary ossification Bone Formation and assess indications for surgery. Injured centers can appear in the fetus as early Page E1 animals should be closely observed dur as 28 days after conception1 (TABLE 1). Anatomy of the Growth ing the period of rapid growth. Growth of the epiphysis arises from two Plate areas: (1) the vascular reserve zone car Page E2 Bone Formation tilage, which is responsible for growth of Physiology of the Growth Bone is formed by transformation of con the epiphysis toward the joint, and (2) the Plate nective tissue (intramembranous ossifica epiphyseal plate, which is responsible for Page E4 tion) and replacement of a cartilaginous growth in bone length.3 The epiphyseal 1 Growth Plate Closure model (endochondral ossification). -

Epiphyseal Photopenia Associated with Metaphyseal Osteomyelitis and Subperiosteal Abscess
Epiphyseal Photopenia Associated with Metaphyseal Osteomyelitis and Subperiosteal Abscess Patrice K. Rehm and John Delahay Division ofNuclear Medicine, Departments ofRadiology and Orthopedic Surgery, Georgetown University Hospital, Washington, DC fevers and knee pain necessitating surgical exploration. An abscess We present a case of metaphysealosteomyelitis in a child where was present, with a small amount of purulence within the bone, as bone scintigraphy demonstrated photopenia of the distal femoral well as copious purulent material beneaththe metaphysealperios epiphysis in the absence of infection of the epiphysis or the joint space. A subsequent bone scan demonstrated evolution of the teum medially, neither being under pressure. No effusion or vascular compromise of the epiphysis due to the metaphyseal purulence was found within the joint or the epiphysis. Copious osteomyelitis complicated by subperiosteal abscess. We discuss irrigation was performed and drains were left in place. Antibiotic the mechanisms and implications of photopenia in the setting of therapy was changed to intravenous penicillin. Gram stain and acute bone and joint infection. cultures of the purulent sitessubsequentlywere positive for Group Key Words epiphyseal photopenia; metaphyseal osteomyelitis; A strep and the epiphysis andjoint sites were negative. subperiostealabscess; bone scintigraphy Becauseof spikingfeversandcontinuedkneepain, the patient J Nuci Med 1998 391084-1086 underwent a second surgical exploration and debridement on the fifth hospital day. Purulent material was found within the knee joint and within the metaphysis, neither being under pressure. Photopeniaonbonescintigraphyintheclinicalsettingofacute The child defervesced and completed a 6-wk course of intrave bone and joint infection is well recognized. It may occur by a nous ceftriaxone. A three-phase bone scan 1 mo after onset variety of mechanisms, all related to alterations in blood flow revealed increased activity of the distal femoral epiphysis in and delivery ofthe radiotracer secondary to infection. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Bone Cartilage Dense Fibrous CT (Tendons & Nonelastic Ligaments) Dense Elastic CT (Elastic Ligaments)
Chapter 6 Content Review Questions 1-8 1. The skeletal system consists of what connective tissues? Bone Cartilage Dense fibrous CT (tendons & nonelastic ligaments) Dense elastic CT (elastic ligaments) List the functions of these tissues. Bone: supports the body, protects internal organs, provides levers on which muscles act, store minerals, and produce blood cells. Cartilage provides a model for bone formation and growth, provides a smooth cushion between adjacent bones, and provides firm, flexible support. Tendons attach muscles to bones and ligaments attach bone to bone. 2. Name the major types of fibers and molecules found in the extracellular matrix of the skeletal system. Collagen Proteoglycans Hydroxyapatite Water Minerals How do they contribute to the functions of tendons, ligaments, cartilage and bones? The collagen fibers of tendons and ligaments make these structures very tough, like ropes or cables. Collagen makes cartilage tough, whereas the water-filled proteoglycans make it smooth and resistant. As a result, cartilage is relatively rigid, but springs back to its original shape if it is bent or slightly compressed, and it is an excellent shock absorber. The extracellular matrix of bone contains collagen and minerals, including calcium and phosphate. Collagen is a tough, ropelike protein, which lends flexible strength to the bone. The mineral component gives the bone compression (weight-bearing) strength. Most of the mineral in the bone is in the form of hydroxyapatite. 3. Define the terms diaphysis, epiphysis, epiphyseal plate, medullary cavity, articular cartilage, periosteum, and endosteum. Diaphysis – the central shaft of a long bone. Epiphysis – the ends of a long bone. Epiphyseal plate – the site of growth in bone length, found between each epiphysis and diaphysis of a long bone and composed of cartilage. -

The Histology of Epiphyseal Union in Mammals
J. Anat. (1975), 120, 1, pp. 1-25 With 49 figures Printed in Great Britain The histology of epiphyseal union in mammals R. WHEELER HAINES* Visiting Professor, Department of Anatomy, Royal Free Hospital School of Medicine, London (Accepted 11 November 1974) INTRODUCTION Epiphyseal union may be defined as beginning with the completion of the first mineralized bridge between epiphyseal and diaphyseal bone and ending with the complete disappearance of the cartilaginous epiphyseal plate and its replacement by bone and marrow. The phases have been described by Sidhom & Derry (1931) and many others from radiographs, but histological material showing union in progress is rare, probably because of the rapidity with which union, once begun, comes to completion (Stephenson, 1924; Dawson, 1929). Dawson (1925, 1929) described the histology of 'lapsed union' in rats, where the larger epiphyses at the 'growing ends' of the long bones remain un-united through- out life. He and Becks et al. (1948) also discussed the early and complete type of union found at the distal end of the humerus in the rat. Here a single narrow per- foration pierced the cartilaginous plate near the olecranon fossa and later spread to destroy the whole plate. Lassila (1928) described a different type of union in the metatarsus of the calf, with multiple perforations of the plate. Apart from a few notes on human material (Haines & Mohiuddin, 1960, 1968), nothing else seems to have been published on the histology of union in mammals. In this paper more abundant material from dog and man is presented and will serve as a basis for discussion of the main features of the different types of union. -
Hand Bone Age: a Digital Atlas of Skeletal Maturity
V. Gilsanz/O. Ratib · Hand Bone Age Vicente Gilsanz · Osman Ratib Hand Bone Age A Digital Atlas of Skeletal Maturity With 88 Figures Vicente Gilsanz, M.D., Ph.D. Department of Radiology Childrens Hospital Los Angeles 4650 Sunset Blvd., MS#81 Los Angeles, CA 90027 Osman Ratib, M.D., Ph.D. Department of Radiology David Geffen School of Medicine at UCLA 100 Medical Plaza Los Angeles, CA 90095 This eBook does not include ancillary media that was packaged with the printed version of the book. ISBN 3-540-20951-4 Springer-Verlag Berlin Heidelberg New York Library of Congress Control Number: 2004114078 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer-Verlag. Violations are liable to prosecution under the German Copyright Law. Springer-Verlag Berlin Heidelberg New York Springer is a part of Springer Science+Business Media http://www.springeronline.com A Springer-Verlag Berlin Heidelberg 2005 Printed in Germany The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from therelevantprotectivelawsandregulationsandthereforefreeforgeneraluse. Product liability: The publishers cannot guarantee the accuracy of any information about the application of operative techniques and medications contained in this book. -

Morphological Studies on the Resistance of Cartilage to Invasion by Osteosarcoma Cells in Vitro and in Vivo"
[CANCER RESEARCH 38, 277-287. February 1978] Morphological Studies on the Resistance of Cartilage to Invasion by Osteosarcoma Cells in Vitro and in Vivo" Klaus E. Kuettner,2 Bendicht U. Pauli, and Lawrence Soble Departments ol Biochemistry [K. E. K], Pathology [B. U. P], and Orthopedic Surgery [K. E. K., L. S.¡,Rush Medical College and Rush College of Health Sciences, Rush Presbyterian-St. Luke's Medical Center, Chicago, Illinois 60612 ABSTRACT repair, and vascularization of tissues during embryogenesis or histogenesis. Tissues such as postnatal hyaline cartilage, Cartilage-bone expiants from human ribs and phalan however, are rarely invaded by inflammatory or neoplastic ges were simultaneously cultured on Millipore mem cells and resist invasion by capillary sprouts (2, 5, 30, 32, branes with either human osteosarcoma cells (TE-85) or 33). The absence of an intrinsic capillary blood supply and human primary foreskin fibroblasts. The explant-tumor the property of cartilage by which it resists penetration by cell interfaces were histologically compared with biopsies capillary endothelial sprouts has been shown to be at least from an osteogenic sarcoma. In both specimens, bone partially mediated by biologically active compounds that spicules were embedded in tumor cell clusters. Irregular can be extracted from hyaline cartilage by guanidinium ities and lacunae in the spicule surfaces with closely hydrochloride (5, 34). These cartilage-derived substances attached tumor cells were considered morphological evi dramatically decrease the proliferation of endothelial cells dence of direct bone erosion by osteosarcoma cells. In in vitro (4, 10). It has been established that the bioactivity contrast to bone, articular and epiphyseal cartilage re within these cartilage extracts resides in molecules with a sisted tumor invasion. -
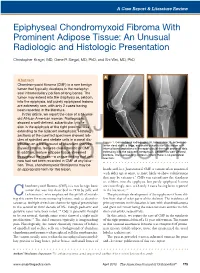
Epiphyseal Chondromyxoid Fibroma with Prominent Adipose Tissue: an Unusual Radiologic and Histologic Presentation
A Case Report & Literature Review Epiphyseal Chondromyxoid Fibroma With Prominent Adipose Tissue: An Unusual Radiologic and Histologic Presentation Christopher Kragel, MD, Gene P. Siegal, MD, PhD, and Shi Wei, MD, PhD A B Abstract Chondromyxoid fibroma (CMF) is a rare benign tumor that typically develops in the metaphy- seal intramedullary portion of long bones. The tumor may extend into the diaphysis or, seldom, into the epiphysis, but purely epiphyseal lesions are extremely rare, with only 2 cases having been reported in the literature. In this article, we report the case of a 51-year- old African American woman. Radiographs showed a well-defined, subarticular lytic le- sion in the epiphysis of the right proximal tibia extending to the adjacent metaphysis.AJO Histologic sections of the curetted specimen showed lob- ules of spindled and stellate cells in a zonal dis- Figure 1. Conventional radiographs (A, lateral view; B, anteropos- tribution on a background of abundant chondro- terior view) show a large, expansile subarticular lytic lesion with myxoid stroma, features characteristic of CMF. internal bony septations in the epiphysis of the right proximal tibia In addition, mature adipose tissue streamed extending into the adjacent metaphysis. Lesion has well-defined borders. The surrounding cortex is intact; there is no periosteal throughout the lesion—a unique finding that until reaction. DOnow had not been recorded NOT in CMF at any loca- COPY tion. Thus, chondromyxoid fibrolipoma may be an appropriate term for this lesion. hands and -

Legg-Calve-Perthes Disease of The
Montefiore Pediatric Orthopedic and Scoliosis Center Children’s Hospital at Montefiore Norman Otsuka MD – Eric Fornari MD Jacob Schulz MD – Jaime Gomez MD – Christine Moloney PA 3400 Bainbridge Avenue, 6th Fl, Bronx, NY 10467 phone 718 920 2060 / fax 718 920 7799 1250 Waters Place, 11th Fl, Bronx, NY 10461 Slipped Capital Femoral Epiphysis (SCFE) What is Slipped Capital Femoral Epiphysis (SCFE)? SCFE is a condition of the hip that usually affects adolescents, in which the epiphysis (growth plate) of the femur (thighbone) becomes separated from the rest of the bone. The epiphysis or growth plate is located at the top of the femur, and the femoral head will usually slip backward and inward in SCFE. A slip occurs when the shearing stress exerted on the femoral head is greater than the resistance provided by the mechanical stability of the growth plate (physis). SCFE is one of the most common orthopaedic hip condition affecting adolescents. What causes Slipped Capital Femoral Epiphysis? There are many theories as to the cause of SCFE, however it is believed be caused by both mechanical as well as constitutional factors. Most likely, SCFE is caused by multiple factors (multifactorial) including local trauma, obesity overcoming the physeal plate (growth plate), inflammatory factors, and possible endocrine abnormalities (increased incidence seen in hypothyroidism, panhypopituitaryism, renal osteodystrophy). Obesity seems to be the strongest risk factor for SCFE, and it is believed that the child’s increased weight causes excessive mechanical stress on the physis (growth plate). Many studies have shown that SCFE tends to occur during an adolescents’ rapid growth spurt, as the growth plate appears to be most vulnerable to shear stress and injury at this time. -

Long Bone Growth and Evolution Revealed by Three-Dimensional Imaging
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1910 Long bone growth and evolution revealed by three-dimensional imaging JORDI ESTEFA ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-513-0885-2 UPPSALA urn:nbn:se:uu:diva-405974 2020 Dissertation presented at Uppsala University to be publicly examined in Lindahlsalen, EBC, Uppsala, Monday, 20 April 2020 at 10:00 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Associate Professor Holly Woodward (Anatomy and Cell Biology, Oklahoma State University, Center for Health Sciences, Tulsa, USA). Abstract Estefa, J. 2020. Long bone growth and evolution revealed by three-dimensional imaging. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1910. 52 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-513-0885-2. Propagation phase-contrast synchrotron radiation microtomography is a non-destructive method used for studying histology in three dimensions (3D). Using it, the 3D organization of the diaphyseal cortical vascularization in the humerus of two seymouriamorphs was analyzed in this thesis. Their vascularization suggests a combination of active growth and a long pre- reproductive period, an intermediate condition between that of Devonian tetrapods and early amniotes, reflecting a gradual change in evolution. The focus of the thesis then shifts to the metaphysis of long bones. The latter possesses complex 3D structures difficult to capture in 2D images. Observations in extant tetrapods have shown that hematopoiesis in long-bones requires the presence of tubular marrow processes opening onto an open medullary cavity with a centralized vascular system. -
Surgical Technique for Conventional Instrumentation
Surgical technique for conventional instrumentation EXTREME® Cementless reconstruction AMPLITUDE femoral stem Design of the EXTREME® femoral stem • The EXTREME® stem product line has been designed for revision and extensive reconstruction cases. • This cementless, lockable femoral stem allows independent adjustment and customised fit of the diaphysis and metaphyseal/epiphyseal components to optimise bone anchoring and joint stability. • For femur reconstruction indications, the EXTREME® stem’s modularity allows the following elements to be adapted to the patient: - Length - Diaphysis diameter - Diaphysis curvature - Metaphyseal filling - Metaphyseal torsion - Extramedullary anteversion. • The two main components of the EXTREME® stem are assembled with a screw and two Morse tapers (small-angle taper technology). • The quality of the impaction of the various modular components can be verified any time during the procedure with the provided instrumentation set. 3 Recommendations ITISNECESSARYTOVERIFYTHEQUALITYOFTHEIMPACTIONBETWEEN THEMONOBLOCKEPIPHYSIS/METAPHYSISANDTHEDIAPHYSEALNAIL. • Neverstriketheassemblyscrewduringimpaction.Thisscrewmustbetightenedusingthe torquewrenchprovidedintheAMPLITUDEinstrumentation. Reminder: The purpose of this surgical technique description is to provide instructions on how to use the instrumentation properly. The surgeon is fully responsible for the indication, surgical approach, surgical technique and postoperative protocol. IMPORTANT: Flexible reamers will be needed in addition to the instrumentation set.