Kato, H. Ossification Pattern of the Vertebral Epiphyses in the Southern
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intervertebral and Epiphyseal Fusion in the Postnatal Ontogeny of Cetaceans and Terrestrial Mammals
J Mammal Evol DOI 10.1007/s10914-014-9256-7 ORIGINAL PAPER Intervertebral and Epiphyseal Fusion in the Postnatal Ontogeny of Cetaceans and Terrestrial Mammals Meghan M. Moran & Sunil Bajpai & J. Craig George & Robert Suydam & Sharon Usip & J. G. M. Thewissen # Springer Science+Business Media New York 2014 Abstract In this paper we studied three related aspects of the Introduction ontogeny of the vertebral centrum of cetaceans and terrestrial mammals in an evolutionary context. We determined patterns The vertebral column provides support for the body and of ontogenetic fusion of the vertebral epiphyses in bowhead allows for flexibility and mobility (Gegenbaur and Bell whale (Balaena mysticetus) and beluga whale 1878;Hristovaetal.2011; Bruggeman et al. 2012). To (Delphinapterus leucas), comparing those to terrestrial mam- achieve this mobility, individual vertebrae articulate with each mals and Eocene cetaceans. We found that epiphyseal fusion other through cartilaginous intervertebral joints between the is initiated in the neck and the sacral region of terrestrial centra and synovial joints between the pre- and post- mammals, while in recent aquatic mammals epiphyseal fusion zygapophyses. The mobility of each vertebral joint varies is initiated in the neck and caudal regions, suggesting loco- greatly between species as well as along the vertebral column motor pattern and environment affect fusion pattern. We also within a single species. Vertebral column mobility greatly studied bony fusion of the sacrum and evaluated criteria used impacts locomotor style, whether the animal is terrestrial or to homologize cetacean vertebrae with the fused sacrum of aquatic. In aquatic Cetacea, buoyancy counteracts gravity, and terrestrial mammals. We found that the initial ossification of the tail is the main propulsive organ (Fish 1996;Fishetal. -

REVIEW ARTICLE Interactions Between GH, IGF-I, Glucocorticoids
0031-3998/02/5202-0137 PEDIATRIC RESEARCH Vol. 52, No. 2, 2002 Copyright © 2002 International Pediatric Research Foundation, Inc. Printed in U.S.A. REVIEW ARTICLE Interactions between GH, IGF-I, Glucocorticoids, and Thyroid Hormones during Skeletal Growth HELEN ROBSON, THOMAS SIEBLER, STEPHEN M. SHALET, AND GRAHAM R. WILLIAMS Department of Clinical Research [H.R.], Department of Endocrinology [S.M.S.], Christie Hospital National Health Service Trust, Manchester, U.K.; University Children’s Hospital, University of Leipzig, Leipzig, Germany [T.S.]; IC Molecular Endocrinology Group, Division of Medicine and MRC Clinical Sciences Centre, Faculty of Medicine, Imperial College School of Science Technology and Medicine, Hammersmith Hospital, London, U.K. [G.R.W.] ABSTRACT Linear growth occurs during development and the childhood Abbreviations years until epiphyseal fusion occurs. This process results from 5'-DI, 5'-iodothyronine deiodinase endochondral ossification in the growth plates of long bones and FGF, fibroblast growth factor is regulated by systemic hormones and paracrine or autocrine FGFR, fibroblast growth factor receptor factors. The major regulators of developmental and childhood GC, glucocorticoid growth are GH, IGF-I, glucocorticoids, and thyroid hormone. GHR, GH receptor Sex steroids are responsible for the pubertal growth spurt and GR, glucocorticoid receptor epiphyseal fusion. This review will consider interactions between HSPG, heparan sulfate proteoglycan GH, IGF-I, glucocorticoids, and thyroid hormone during linear 11HSD, 11-hydroxysteroid dehydrogenase growth. It is well known from physiologic and clinical studies IGF-IR, IGF-I receptor that these hormones interact at the level of the hypothalamus and IGFBP, IGF binding protein pituitary. Interacting effects on peripheral tissues such as liver are Ihh, Indian hedgehog also well understood, but we concentrate here on the epiphyseal JAK-2, Janus-activated kinase-2 growth plate as an important and newly appreciated target organ PTHrP, PTH-related peptide for convergent hormone action. -

The Epiphyseal Plate: Physiology, Anatomy, and Trauma*
3 CE CREDITS CE Article The Epiphyseal Plate: Physiology, Anatomy, and Trauma* ❯❯ Dirsko J. F. von Pfeil, Abstract: This article reviews the development of long bones, the microanatomy and physiology Dr.med.vet, DVM, DACVS, of the growth plate, the closure times and contribution of different growth plates to overall growth, DECVS and the effect of, and prognosis for, traumatic injuries to the growth plate. Details on surgical Veterinary Specialists of Alaska Anchorage, Alaska treatment of growth plate fractures are beyond the scope of this article. ❯❯ Charles E. DeCamp, DVM, MS, DACVS athologic conditions affecting epi foramen. Growth factors and multipotent Michigan State University physeal (growth) plates in imma stem cells support the formation of neo ture animals may result in severe natal bone consisting of a central marrow P 2 orthopedic problems such as limb short cavity surrounded by a thin periosteum. ening, angular limb deformity, or joint The epiphysis is a secondary ossifica incongruity. Understanding growth plate tion center in the hyaline cartilage forming anatomy and physiology enables practic the joint surfaces at the proximal and distal At a Glance ing veterinarians to provide a prognosis ends of the bones. Secondary ossification Bone Formation and assess indications for surgery. Injured centers can appear in the fetus as early Page E1 animals should be closely observed dur as 28 days after conception1 (TABLE 1). Anatomy of the Growth ing the period of rapid growth. Growth of the epiphysis arises from two Plate areas: (1) the vascular reserve zone car Page E2 Bone Formation tilage, which is responsible for growth of Physiology of the Growth Bone is formed by transformation of con the epiphysis toward the joint, and (2) the Plate nective tissue (intramembranous ossifica epiphyseal plate, which is responsible for Page E4 tion) and replacement of a cartilaginous growth in bone length.3 The epiphyseal 1 Growth Plate Closure model (endochondral ossification). -

Epiphyseal Photopenia Associated with Metaphyseal Osteomyelitis and Subperiosteal Abscess
Epiphyseal Photopenia Associated with Metaphyseal Osteomyelitis and Subperiosteal Abscess Patrice K. Rehm and John Delahay Division ofNuclear Medicine, Departments ofRadiology and Orthopedic Surgery, Georgetown University Hospital, Washington, DC fevers and knee pain necessitating surgical exploration. An abscess We present a case of metaphysealosteomyelitis in a child where was present, with a small amount of purulence within the bone, as bone scintigraphy demonstrated photopenia of the distal femoral well as copious purulent material beneaththe metaphysealperios epiphysis in the absence of infection of the epiphysis or the joint space. A subsequent bone scan demonstrated evolution of the teum medially, neither being under pressure. No effusion or vascular compromise of the epiphysis due to the metaphyseal purulence was found within the joint or the epiphysis. Copious osteomyelitis complicated by subperiosteal abscess. We discuss irrigation was performed and drains were left in place. Antibiotic the mechanisms and implications of photopenia in the setting of therapy was changed to intravenous penicillin. Gram stain and acute bone and joint infection. cultures of the purulent sitessubsequentlywere positive for Group Key Words epiphyseal photopenia; metaphyseal osteomyelitis; A strep and the epiphysis andjoint sites were negative. subperiostealabscess; bone scintigraphy Becauseof spikingfeversandcontinuedkneepain, the patient J Nuci Med 1998 391084-1086 underwent a second surgical exploration and debridement on the fifth hospital day. Purulent material was found within the knee joint and within the metaphysis, neither being under pressure. Photopeniaonbonescintigraphyintheclinicalsettingofacute The child defervesced and completed a 6-wk course of intrave bone and joint infection is well recognized. It may occur by a nous ceftriaxone. A three-phase bone scan 1 mo after onset variety of mechanisms, all related to alterations in blood flow revealed increased activity of the distal femoral epiphysis in and delivery ofthe radiotracer secondary to infection. -

Postnatal Maturation and Radiology of the Growing Spine Sharon E
Neurosurg Clin N Am 18 (2007) 431–461 Postnatal Maturation and Radiology of the Growing Spine Sharon E. Byrd, MDa,b,*, Elizabeth M. Comiskey, MDa,c aRush Medical College, 1653 West Congress Parkway, Chicago, IL 60612, USA bSection of Neuroradiology, Department of Diagnostic Radiology and Nuclear Medicine, Rush University Medical Center, 1653 West Congress Parkway, Chicago, IL 60612, USA cSection of Pediatric Radiology, Department of Diagnostic Radiology and Nuclear Medicine, Rush University Medical Center, 1653 West Congress Parkway, Chicago, IL 60612, USA The spine is part of the supporting framework Anatomy of the body and is composed of vertebrae, discs, The spine consists of osseous and soft tissue and ligaments. It continues to mature postnatally, components that provide support and mobility for with marked changes occurring predominantly in the body and a protective covering for the central the vertebrae during infancy, childhood, and early nervous system. The vertebral column is com- adolescence. Maturation of the spine is not only posed of 7 cervical, 12 thoracic, and 5 lumbar manifested by the ossification process but by vertebrae; the sacrum (composed of 5 fused changes in the shape of the vertebrae, spinal vertebrae that become progressively smaller); curvature, spinal canal, discs, and bone marrow. and the coccyx (3 to 5 rudimentary vertebrae). A The parts of the spine and the maturation process typical vertebra consists of a body and neural can be evaluated by various imaging modalities arch. The neural arch is composed of bilateral such as conventional plain spine imaging (CPSI pedicles, laminae, superior and inferior articulat- [plain spine radiography]), CT, and MRI. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Biology of Bone Repair
Biology of Bone Repair J. Scott Broderick, MD Original Author: Timothy McHenry, MD; March 2004 New Author: J. Scott Broderick, MD; Revised November 2005 Types of Bone • Lamellar Bone – Collagen fibers arranged in parallel layers – Normal adult bone • Woven Bone (non-lamellar) – Randomly oriented collagen fibers – In adults, seen at sites of fracture healing, tendon or ligament attachment and in pathological conditions Lamellar Bone • Cortical bone – Comprised of osteons (Haversian systems) – Osteons communicate with medullary cavity by Volkmann’s canals Picture courtesy Gwen Childs, PhD. Haversian System osteocyte osteon Picture courtesy Gwen Childs, PhD. Haversian Volkmann’s canal canal Lamellar Bone • Cancellous bone (trabecular or spongy bone) – Bony struts (trabeculae) that are oriented in direction of the greatest stress Woven Bone • Coarse with random orientation • Weaker than lamellar bone • Normally remodeled to lamellar bone Figure from Rockwood and Green’s: Fractures in Adults, 4th ed Bone Composition • Cells – Osteocytes – Osteoblasts – Osteoclasts • Extracellular Matrix – Organic (35%) • Collagen (type I) 90% • Osteocalcin, osteonectin, proteoglycans, glycosaminoglycans, lipids (ground substance) – Inorganic (65%) • Primarily hydroxyapatite Ca5(PO4)3(OH)2 Osteoblasts • Derived from mesenchymal stem cells • Line the surface of the bone and produce osteoid • Immediate precursor is fibroblast-like Picture courtesy Gwen Childs, PhD. preosteoblasts Osteocytes • Osteoblasts surrounded by bone matrix – trapped in lacunae • Function -

Bone Cartilage Dense Fibrous CT (Tendons & Nonelastic Ligaments) Dense Elastic CT (Elastic Ligaments)
Chapter 6 Content Review Questions 1-8 1. The skeletal system consists of what connective tissues? Bone Cartilage Dense fibrous CT (tendons & nonelastic ligaments) Dense elastic CT (elastic ligaments) List the functions of these tissues. Bone: supports the body, protects internal organs, provides levers on which muscles act, store minerals, and produce blood cells. Cartilage provides a model for bone formation and growth, provides a smooth cushion between adjacent bones, and provides firm, flexible support. Tendons attach muscles to bones and ligaments attach bone to bone. 2. Name the major types of fibers and molecules found in the extracellular matrix of the skeletal system. Collagen Proteoglycans Hydroxyapatite Water Minerals How do they contribute to the functions of tendons, ligaments, cartilage and bones? The collagen fibers of tendons and ligaments make these structures very tough, like ropes or cables. Collagen makes cartilage tough, whereas the water-filled proteoglycans make it smooth and resistant. As a result, cartilage is relatively rigid, but springs back to its original shape if it is bent or slightly compressed, and it is an excellent shock absorber. The extracellular matrix of bone contains collagen and minerals, including calcium and phosphate. Collagen is a tough, ropelike protein, which lends flexible strength to the bone. The mineral component gives the bone compression (weight-bearing) strength. Most of the mineral in the bone is in the form of hydroxyapatite. 3. Define the terms diaphysis, epiphysis, epiphyseal plate, medullary cavity, articular cartilage, periosteum, and endosteum. Diaphysis – the central shaft of a long bone. Epiphysis – the ends of a long bone. Epiphyseal plate – the site of growth in bone length, found between each epiphysis and diaphysis of a long bone and composed of cartilage. -

The Histology of Epiphyseal Union in Mammals
J. Anat. (1975), 120, 1, pp. 1-25 With 49 figures Printed in Great Britain The histology of epiphyseal union in mammals R. WHEELER HAINES* Visiting Professor, Department of Anatomy, Royal Free Hospital School of Medicine, London (Accepted 11 November 1974) INTRODUCTION Epiphyseal union may be defined as beginning with the completion of the first mineralized bridge between epiphyseal and diaphyseal bone and ending with the complete disappearance of the cartilaginous epiphyseal plate and its replacement by bone and marrow. The phases have been described by Sidhom & Derry (1931) and many others from radiographs, but histological material showing union in progress is rare, probably because of the rapidity with which union, once begun, comes to completion (Stephenson, 1924; Dawson, 1929). Dawson (1925, 1929) described the histology of 'lapsed union' in rats, where the larger epiphyses at the 'growing ends' of the long bones remain un-united through- out life. He and Becks et al. (1948) also discussed the early and complete type of union found at the distal end of the humerus in the rat. Here a single narrow per- foration pierced the cartilaginous plate near the olecranon fossa and later spread to destroy the whole plate. Lassila (1928) described a different type of union in the metatarsus of the calf, with multiple perforations of the plate. Apart from a few notes on human material (Haines & Mohiuddin, 1960, 1968), nothing else seems to have been published on the histology of union in mammals. In this paper more abundant material from dog and man is presented and will serve as a basis for discussion of the main features of the different types of union. -

The Genetic Transformation of Bone Biology
Downloaded from genesdev.cshlp.org on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW The genetic transformation of bone biology Gerard Karsenty1 Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, Texas 77030 The skeleton, like every organ, has specific developmen- condensations differentiate into chondrocytes forming tal and functional characteristics that define its identity the “cartilage anlagen” of the future bones. In the pe- in biologic and pathologic terms. Skeleton is composed riphery of the anlage, cells from the perichondrium dif- of multiple elements of various shapes and origins spread ferentiate into osteoblasts, while the periphery of the throughout the body. Most of these skeletal elements are anlage become hypertrophic. Eventually, the matrix sur- formed by two different tissues, cartilage and bone, and rounding these hypertrophic chondrocytes calcifies and each of these two tissues has its own specific cell types: blood vessel invasion of the calcified cartilage brings in the chondrocyte in cartilage, and the osteoblast and os- osteoblasts ∼14.5–15.5 dpc (Horton 1993; Erlebacher et teoclast in bone. Finally, each of these cell types has its al. 1995). Once a bone matrix is deposited the bone mar- own differentiation pathway, physiological functions, row forms and the first osteoclasts appear (Hofstetter et and therefore pathological conditions. The complexity of al. 1995). Thus, sequential appearance of a cartilage an- this organ in terms of developmental biology, physiol- lage, calcified cartilage, and then bona fide bone charac- ogy, and pathology, along with the multitude of impor- terizes the endochondral ossification. Regardless of the tant conceptual advances in our understanding of skel- mode of ossification, osteoblast differentiation precedes etal biology, are such that it has become impossible to osteoclast differentiation. -

Pathophysiology of Heterotopic Ossification
PATHOPHYSIOLOGY Pathophysiology of Heterotopic Ossifi cation Michael E. Zychowicz Heterotopic ossifi cation (HO) is a pathologic condition that Up to 35% of those patients with an SCI will develop leads to the development of bone within nonosseous soft a limitation in range of motion of the affected body part tissues. A common site for HO development is at the hip. (Shehab et al., 2002 ). Between 70% and 97% of patients The bone that forms is believed to develop through stimula- will have the hip as their primary site of HO develop- tion by cellular mediators and altered neurovascular signal- ment within 2–3 weeks following SCIs. Nearly 8% of ing. Heterotopic ossifi cation can be a debilitating disease patients will go on to develop ankylosis at the hip (Teasell et al., 2010 ). Following an SCI, there appears to leading to pain, edema, and stiffness. This only compounds be a relationship with the development of HO and expe- already-debilitating comorbid conditions such as a spinal riencing muscle spasticity, a fracture, limited range of cord injury, head injury, or trauma. Several factors, includ- motion or greater than 2 weeks’ loss of consciousness ing prostaglandin E2, bone morphogenetic protein, and (Aubut, Mehta, Cullen, & Teasell, 2011). the infl ammatory process, are believed to contribute to In patients with a closed brain injury, HO occurs in the development of HO. The full scope of pathophysiology approximately 10%–20% of patients. Nearly 10% of contributing to HO is not fully understood. those who develop HO following a closed brain injury will also develop signifi cantly limited range of motion at the affected joint (Shehab et al., 2002 ). -
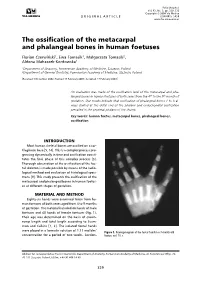
The Ossification of the Metacarpal and Phalangeal Bones in Human Foetuses
Folia Morphol. Vol. 63, No. 3, pp. 329–332 Copyright © 2004 Via Medica O R I G I N A L A R T I C L E ISSN 0015–5659 www.fm.viamedica.pl The ossification of the metacarpal and phalangeal bones in human foetuses Florian Czerwiński1, Ewa Tomasik1, Małgorzata Tomasik2, Aldona Mahaczek-Kordowska1 1Department of Anatomy, Pomeranian Academy of Medicine, Szczecin, Poland 2Department of General Dentistry, Pomeranian Academy of Medicine, Szczecin, Poland [Received 4 November 2002; Revised 17 February 2004; Accepted 17 February 2004] An evaluation was made of the ossification level of the metacarpal and pha- langeal bones in human foetuses of both sexes from the 4th to the 9th month of gestation. Our results indicate that ossification of phalangeal bones 1 to 5 al- ways started at the distal end of the phalanx and endochondral ossification prevailed in the proximal phalanx of the thumb. Key words: human foetus, metacarpal bones, phalangeal bones, ossification INTRODUCTION Most human skeletal bones are ossified on a car- tilaginous base [5, 14]. This is a complex process pro- gressing dynamically in time and ossification consti- tutes the final phase of this complex process [3]. Thorough observation of the ossification of the foe- tal skeleton is made possible by means of the radio- logical method and evaluation of histological speci- mens [9]. This study presents the ossification of the metacarpal and phalangeal bones in human foetus- es at different stages of gestation. MATERIAL AND METHOD Eighty-six hands were examined taken from hu- man foetuses of both sexes aged from 4 to 9 months of gestation.