Appendix 1 Development of the Ausplots Fauna Survey Protocol
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Broken Hill Complex
Broken Hill Complex Bioregion resources Photo Mulyangarie, DEH Broken Hill Complex The Broken Hill Complex bioregion is located in western New South Wales and eastern South Australia, spanning the NSW-SA border. It includes all of the Barrier Ranges and covers a huge area of nearly 5.7 million hectares with approximately 33% falling in South Australia! It has an arid climate with dry hot summers and mild winters. The average rainfall is 222mm per year, with slightly more rainfall occurring in summer. The bioregion is rich with Aboriginal cultural history, with numerous archaeological sites of significance. Biodiversity and habitat The bioregion consists of low ranges, and gently rounded hills and depressions. The main vegetation types are chenopod and samphire shrublands; casuarina forests and woodlands and acacia shrublands. Threatened animal species include the Yellow-footed Rock- wallaby and Australian Bustard. Grazing, mining and wood collection for over 100 years has led to a decline in understory plant species and cover, affecting ground nesting birds and ground feeding insectivores. 2 | Broken Hill Complex Photo by Francisco Facelli Broken Hill Complex Threats Threats to the Broken Hill Complex bioregion and its dependent species include: For Further information • erosion and degradation caused by overgrazing by sheep, To get involved or for more information please cattle, goats, rabbits and macropods phone your nearest Natural Resources Centre or • competition and predation by feral animals such as rabbits, visit www.naturalresources.sa.gov.au -

Central Eyre Iron Project Environmental Impact Statement
Central Eyre Iron Project Environmental Impact Statement EIS REFERENCES REFERENCES COPYRIGHT Copyright © Iron Road Limited, 2015 All rights reserved This document and any related documentation is protected by copyright owned by Iron Road Limited. The content of this document and any related documentation may only be copied and distributed for the purposes of section 46B of the Development Act, 1993 (SA) and otherwise with the prior written consent of Iron Road Limited. DISCLAIMER Iron Road Limited has taken all reasonable steps to review the information contained in this document and to ensure its accuracy as at the date of submission. Note that: (a) in writing this document, Iron Road Limited has relied on information provided by specialist consultants, government agencies, and other third parties. Iron Road Limited has reviewed all information to the best of its ability but does not take responsibility for the accuracy or completeness; and (b) this document has been prepared for information purposes only and, to the full extent permitted by law, Iron Road Limited, in respect of all persons other than the relevant government departments, makes no representation and gives no warranty or undertaking, express or implied, in respect to the information contained herein, and does not accept responsibility and is not liable for any loss or liability whatsoever arising as a result of any person acting or refraining from acting on any information contained within it. References A ADS 2014, Adelaide Dolphin Sanctuary, viewed January 2014, http://www.naturalresources.sa.gov.au/adelaidemtloftyranges/coast-and-marine/dolphin-sanctuary. Ainslie, RC, Johnston, DA & Offler, EW 1989, Intertidal communities of Northern Spencer Gulf, South Australia, Transactions of the Royal Society of South Australia, Adelaide. -

Phylogenetic Structure of Vertebrate Communities Across the Australian
Journal of Biogeography (J. Biogeogr.) (2013) 40, 1059–1070 ORIGINAL Phylogenetic structure of vertebrate ARTICLE communities across the Australian arid zone Hayley C. Lanier*, Danielle L. Edwards and L. Lacey Knowles Department of Ecology and Evolutionary ABSTRACT Biology, Museum of Zoology, University of Aim To understand the relative importance of ecological and historical factors Michigan, Ann Arbor, MI 48109-1079, USA in structuring terrestrial vertebrate assemblages across the Australian arid zone, and to contrast patterns of community phylogenetic structure at a continental scale. Location Australia. Methods We present evidence from six lineages of terrestrial vertebrates (five lizard clades and one clade of marsupial mice) that have diversified in arid and semi-arid Australia across 37 biogeographical regions. Measures of within-line- age community phylogenetic structure and species turnover were computed to examine how patterns differ across the continent and between taxonomic groups. These results were examined in relation to climatic and historical fac- tors, which are thought to play a role in community phylogenetic structure. Analyses using a novel sliding-window approach confirm the generality of pro- cesses structuring the assemblages of the Australian arid zone at different spa- tial scales. Results Phylogenetic structure differed greatly across taxonomic groups. Although these lineages have radiated within the same biome – the Australian arid zone – they exhibit markedly different community structure at the regio- nal and local levels. Neither current climatic factors nor historical habitat sta- bility resulted in a uniform response across communities. Rather, historical and biogeographical aspects of community composition (i.e. local lineage per- sistence and diversification histories) appeared to be more important in explaining the variation in phylogenetic structure. -

Building Nature's Safety Net 2008
Building Nature’s Safety Net 2008 Progress on the Directions for the National Reserve System Paul Sattler and Martin Taylor Telstra is a proud partner of the WWF Building Nature's Map sources and caveats Safety Net initiative. The Interim Biogeographic Regionalisation for Australia © WWF-Australia. All rights protected (IBRA) version 6.1 (2004) and the CAPAD (2006) were ISBN: 1 921031 271 developed through cooperative efforts of the Australian Authors: Paul Sattler and Martin Taylor Government Department of the Environment, Water, Heritage WWF-Australia and the Arts and State/Territory land management agencies. Head Office Custodianship rests with these agencies. GPO Box 528 Maps are copyright © the Australian Government Department Sydney NSW 2001 of Environment, Water, Heritage and the Arts 2008 or © Tel: +612 9281 5515 Fax: +612 9281 1060 WWF-Australia as indicated. www.wwf.org.au About the Authors First published March 2008 by WWF-Australia. Any reproduction in full or part of this publication must Paul Sattler OAM mention the title and credit the above mentioned publisher Paul has a lifetime experience working professionally in as the copyright owner. The report is may also be nature conservation. In the early 1990’s, whilst with the downloaded as a pdf file from the WWF-Australia website. Queensland Parks and Wildlife Service, Paul was the principal This report should be cited as: architect in doubling Queensland’s National Park estate. This included the implementation of representative park networks Sattler, P.S. and Taylor, M.F.J. 2008. Building Nature’s for bioregions across the State. Paul initiated and guided the Safety Net 2008. -

Approved Conservation Advice for Olearia Pannosa Subsp. Pannosa (Silver Daisy-Bush)
This Conservation Advice was approved by the Delegate of the Minister on 17 December 2013 Approved Conservation Advice for Olearia pannosa subsp. pannosa (silver daisy-bush) (s266B of the Environment Protection and Biodiversity Conservation Act 1999) This Conservation Advice has been developed based on the best available information at the time this Conservation Advice was approved; this includes existing and draft plans, records or management prescriptions for this species. Description Olearia pannosa subsp. pannosa (silver daisy-bush), family Asteraceae, is a spreading undershrub or shrub growing up to 1.5 m high, producing rooting stems that can spread over 10–20 m along the ground. The leaf lamina (blade) is elliptic to ovate, the length usually greater than twice the width and obtuse to acute at the base. The hairs of the lower leaf surface and peduncle are appressed, white to cream or a very pale rusty-brown. Flower heads are white or rarely pale mauve, with a yellow centre (Cooke, 1986; Wisniewski et al., 1987; Cropper, 1993). The silver daisy-bush differs from the velvet Daisy-bush (Olearia pannosa subsp. cardiophylla) in leaf shape and the orientation and colour of hairs on the leaf (Willson & Bignall, 2009). Leaf shape is elliptic to ovate in the former and broad-ovate in the latter; and hairs in the former are appressed and white to cream or a very pale rusty-brown, and in the latter are slightly appressed and buff to rusty-brown (Cooke, 1986) Conservation Status The silver daisy-bush is listed as vulnerable under the name Olearia pannosa subsp. -

Vegetation Change Along a Mediterranean to Arid Zone Bioclimatic Gradient Reveals Scale-Dependent Ecotone Patterning S
Australian Journal of Botany, 2020, 68, 574–586 © CSIRO 2020 10.1071/BT20036_AC Supplementary material Vegetation change along a Mediterranean to arid zone bioclimatic gradient reveals scale-dependent ecotone patterning S. Caddy-RetalicA,B,D, G. M. WardleB, E. J. LeitchA, F. A. McInerneyC and A. J. LoweA ASchool of Biological Sciences and Environment Institute, University of Adelaide, North Terrace, SA 5005, Australia. BSchool of Life and Environmental Sciences, University of Sydney, Sydney, NSW 2006, Australia. CSchool of Physical Sciences and Sprigg Geobiology Centre, University of Adelaide, North Terrace, SA 5005, Australia. DCorresponding author. Email: [email protected] Table S1. Comparison of TREND-Guerin and TREND-AusPlots transect surveys Design TREND-Guerin plots TREND-AusPlots Total plots 85 42 Plot configuration 17 groups of 5 plots Single plots Plot size 900 m2 (30 × 30 m) 1 ha (100 ×100 m) Total area sampled 7.65 ha 42 ha Transect length 550 km 700 km 4.8° latitude 6.2° latitude Environmental 684 mm MAP 818 mm MAP (980–162 mm) gradient (307–991 mm) 4°C MAT (13.4–17.4°C) 7.2°C MAT (13.4–20.6°C) Time of sampling 1 Sep–9 Nov 2011 (all sites) 13–22 Aug 2012 (SATFLB0001-15, SATKAN0001-2) 6–17 Aug 2013 (SATFLB0016-25, SATSTP0001-8) 28 Oct–6 Nov 2014 (SATEYB0001-2, SATFLB0027-28, SATKAN0003-4) Observers GRG EJL, SCR & IF 1 Table S2. TREND site codes and location information including agro-climatic zone and Interim Bioregionalisation of Australia (IBRA) subregion Guerin codes align to those published in (Guerin et al. -

Data on Significant Wilderness Areas in the Alinytjara Wilurara and South Australian Arid Lands NRM Regions
Data on significant wilderness areas in the Alinytjara Wilurara and South Australian Arid Lands NRM Regions Wilderness Advisory Committee November 2014 Acknowledgments The Wilderness Advisory Committee acknowledges the invaluable work of the late Dr Rob Lesslie. His work forms the basis of much of this report, with the Wilderness Advisory Committee holding responsibility for the report. We thank the staff of the Department of Environment, Water and Natural Resources for their assistance, in particular Jason Irving and Ian Sellar. i | Data on significant wilderness areas in the Alinytjara Wilurara and South Australian Arid Lands NRM Regions Contents 1. Purpose of the report 1 2. The significance of wilderness 1 3. Wilderness surveys 3 4. Adequacy of formal protection 3 5. Management principles for the arid environment 4 6. Conclusion 5 Appendix 1. Wilderness Areas of Potential 6 National Significance: description Appendix 2. Climate change priority actions 26 Appendix 3 Maps 28 Map 1 Wilderness Areas of Potential 29 National Significance: Bioregions Map 2 Wilderness Areas of Potential 31 National Significance: Land Ownership Map 3 Wilderness Areas of Potential 33 National Significance: Watercourses and wetlands Map 4 Wilderness Areas of Potential 35 National Significance: Waterpoints Map 5 National Wilderness Inventory 37 Map 6 Wilderness Areas of Potential 39 National Significance: Conservation Area Type Map 7 Bioregional Distribution of Highly 41 Protected Areas (IUCN Category Ia, Ib, II and III) Map 8 Predicted Temperature Increase 42 for South Australia, 2030, 2050 and 2070 Data on significant wilderness areas in the Alinytjara Wilurara and South Australian Arid Lands NRM Regions | ii Left and right image: Nullabor Plains, South Australia 1. -

Approved Conservation Advice for Pleuropappus Phyllocalymmeus (Silver Candles)
This Conservation Advice was approved by the Delegate of the Minister on 17 December 2013 Approved Conservation Advice for Pleuropappus phyllocalymmeus (silver candles) (s266B of the Environment Protection and Biodiversity Conservation Act 1999) This Conservation Advice has been developed based on the best available information at the time this Conservation Advice was approved; this includes existing and draft plans, records or management prescriptions for this species. Description Pleuropappus phyllocalymmeus (silver candles), family Asteraceae, is an annual that grows to 4–15 cm high. Each plant has several stems from the base that are ascending, simple or few branched and pubescent or hairless. Leaves are linear to narrowly lance shaped with a pointed end, 7–12 mm long, 1 mm wide, greyish and cobwebby-hairy. Flowers are compound, 8–20 mm long, 3–5 mm in diameter, with 40–100 capitula, usually solitary, golden and lustrous. Flowering occurs in September to December (State Herbarium of South Australia, 2007). Conservation Status Silver candles is listed as vulnerable under the name Pleuropappus phyllocalymmeus. This species is eligible for listing as vulnerable under the Environment Protection and Biodiversity Conservation Act 1999 (Cwlth) (EPBC Act) as, prior to the commencement of the EPBC Act, it was listed as vulnerable under Schedule 1 of the Endangered Species Protection Act 1992 (Cwlth). The species is also listed as vulnerable under the National Parks and Wildlife Act 1972 (South Australia). Distribution and Habitat Silver candles is endemic to South Australia where it occurs on Eyre Peninsula and Yorke Peninsula (ALA, 2013). The species has a sporadic distribution, although it is locally common (Short, 1983). -

Biogeographical Variation in the Potential Effectiveness of Prescribed Fire in South-Eastern Australia Owen F
University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers Faculty of Science, Medicine and Health 2015 Biogeographical variation in the potential effectiveness of prescribed fire in south-eastern Australia Owen F. Price University of Wollongong, [email protected] Trent D. Penman University of Melbourne, [email protected] Ross A. Bradstock University of Wollongong, [email protected] Mathias M. Boer Universty of Western Sydney Hamish Clarke NSW Office ofn E vironment and Heritage Publication Details Price, O. F., Penman, T. D., Bradstock, R. A., Boer, M. M. & Clarke, H. (2015). Biogeographical variation in the potential effectiveness of prescribed fire in south-eastern Australia. Journal of Biogeography, 42 (11), 2234-2245. Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Biogeographical variation in the potential effectiveness of prescribed fire in south-eastern Australia Abstract Aim Prescribed fire is a common land management for reducing risks from unplanned fires. However, the universality of such effectiveness remains uncertain due to biogeographical variation in fuel types, climatic influences and fire regimes. Here, we explore biogeographical patterns in the effectiveness of prescribed fire by calculating leverage (the reduction in unplanned area burnt resulting from recent previous area burnt) across south-eastern Australia over a 25 year period. Location The 30 bioregions of south-eastern Australia. Methods We quantified leverage in each bioregion from fire records from 1975-2009, controlling for variation in annual weather. We also identified potential drivers of variation in leverage by relating the bioregional leverage values to measures of fuel type and growth, climate, and weather extremes. -

(SIS) – 2013 Version
Information Sheet on EAA Flyway Network Sites Information Sheet on EAA Flyway Network Sites (SIS) – 2013 version Available for download from http://www.eaaflyway.net/about/the-flyway/flyway-site-network/ Categories approved by Second Meeting of the Partners of the East Asian-Australasian Flyway Partnership in Beijing, China 13-14 November 2007 - Report (Minutes) Agenda Item 3.13 1. Name and contact details of the compiler of this form: Full name: Arkellah Irving EAAF SITE CODE FOR OFFICE USE ONLY: Institution/agency: Adelaide International Bird Sanctuary/ Department of Environment, Water and Natural Resources. Address : GPO Box 1047, Adelaide, SA 5001 E A A F 1 3 1 Telephone: (08) 8463 7131 Mobile: 0409 426 371 Fax numbers: N/A E-mail address: [email protected] 2. Date this sheet was completed: 2/09/2016 3. Country: Australia 4. Name of the Flyway Network site: Adelaide International Bird Sanctuary 1 Information Sheet on EAA Flyway Network Sites 5. Map of site: 6. Geographical coordinates (latitude/longitude, in decimal degrees): Due to the narrow ‘boomerang’-like shape of the proposed site it was not possible to distinguish a centre point. Instead three points in a triangular shape have been selected to provide geographical coordinates. The geographical coordinates are taken from the northernmost boundary of the specified area of interest above Clinton Conservation Park and the southernmost points of the easten boundary below the Dry Creek 2 Information Sheet on EAA Flyway Network Sites wetlands and the western boundarary below the Price saltfields. The coordinates have been provided in degrees, minutes, seconds: Northern boundary - ’ ’ South-western boundary - ’ ’ South-eastern boundary - ’ ‘ 7. -
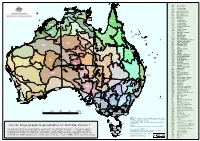
Interim Biogeographic Regionalisation for Australia, Version 7 Data Used Are Assumed to Be Correct As Received from the Data Suppliers
ARC Arnhem Coast ARP Arnhem Plateau TIW AUA Australian Alps AVW Avon Wheatbelt DARWIN BBN Brigalow Belt North ARC BBS Brigalow Belt South BEL Ben Lomond ITI DAC PCK ARP BHC Broken Hill Complex CEA BRT Burt Plain CAR Carnarvon ARC CEA Central Arnhem DAB CYP CEK Central Kimberley CER Central Ranges NOK VIB CHC Channel Country CMC Central Mackay Coast GUC COO Coolgardie GFU STU COP Cobar Peneplain COS Coral Sea CEK CYP Cape York Peninsula OVP DAB Daly Basin DAC Darwin Coastal DAL WET GUP EIU DAL Dampierland DEU Desert Uplands DMR Davenport Murchison Ranges COS DRP Darling Riverine Plains DMR TAN EIU Einasleigh Uplands MII ESP Esperance Plains GSD EYB Eyre Yorke Block FIN Finke FLB Flinders Lofty Block CMC FUR Furneaux BRT GAS Gascoyne PIL DEU GAW Gawler MGD BBN GES Geraldton Sandplains GFU Gulf Fall and Uplands MAC GID Gibson Desert LSD GID GSD Great Sandy Desert GUC Gulf Coastal GUP Gulf Plains CAR GAS CER FIN CHC GVD Great Victoria Desert HAM Hampton ITI Indian Tropical Islands SSD JAF Jarrah Forest KAN Kanmantoo KIN King GVD LSD Little Sandy Desert STP BBS MUR SEQ MAC MacDonnell Ranges MUL BRISBANE MAL Mallee MDD Murray Darling Depression YAL MGD GES Mitchell Grass Downs STP MII Mount Isa Inlier MUL Mulga Lands NUL MUR Murchison NAN Nandewar GAW NET NCP Naracoorte Coastal Plain SWA COO NAN NET New England Tablelands AVW HAM BHC DRP NNC NSW North Coast FLB NOK Northern Kimberley PERTH COP NSS NSW South Western Slopes MDD NNC NUL Nullarbor MAL EYB OVP Ord Victoria Plain PCK Pine Creek JAF ESP SYB PIL Pilbara ADELAIDE SYDNEY PSI PSI Pacific -

Adec Preview Generated PDF File
DOI: 10.18195/issn.0312-3162.21(4).2003.367-370 Records of the Westem Australian Museum 21: 367-370 (2003). Short communication The relationship between eastern and western populations of the Heath Rat, Pseudomys shortridgei (Rodentia: Muridae) N.K. Cooper!, T. BertozzP, Alexander Baynesl and R.J. Teale3 I Western Australian Museum, Francis Street, Perth, Western Australia 6()(}O Australia 2 Evolutionary Biology Unit, South Australian Museum, North Terrace, Adelaide, South Australia SO()(}, Australia 3 BlOTA Environmental Sciences, 186 Scarborough Beach Road, Mount Hawthorn, Western Australia 6016, Australia The Heath Rat, Pseudomys shortridgei (Thomas, species is classified as Mammals that are 1907), has a wide but disjunct range across southern Vulnerable. Australia, occurring in heaths and shrublands in In a review of the fauna recorded from the western Victoria, southern South Australia and Fitzgerald Biosphere Reserve, Chapman et al. Western Australia (Cockburn 1995). The holotype (unpublished) compiled 77 records of this species of P. sllOrtridgei (Natural History Museum, London, (excluding the nine specimens in the Western no. 6.8.1.73) was collected by G.c. Shortridge on 27 Australian Museum collection). Vegetation of the Apr 1906 from Woyerling, east of Pingelly, Western sites varied, as did soil type. The predominant Australia (Tate 1951) (see Figure 1). Shortridge vegetation was shrub mallee over either heath or (1936) recorded the locality as "Woyaline Wells scrub over sedges. Sedges are thought to be an (source of the Avon River)". In Western Australia it important dietary component (A. Sanders personal is presently known only from Fitzgerald River communication 2003). Some individuals were also National Park, Lake Magenta Reserve, Dragon trapped in shrublands on granites and low forest Rocks Reserve, Hyden area and Ravensthorpe principally comprising Eucalyptus gardneri (specimens in the Western Australian Museum ravenst1lOrpensis.