Time Asymmetry in the Palaeobiogeographic History of Species
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Laboratory Primate Newsletter
LABORATORY PRIMATE NEWSLETTER Vol. 45, No. 3 July 2006 JUDITH E. SCHRIER, EDITOR JAMES S. HARPER, GORDON J. HANKINSON AND LARRY HULSEBOS, ASSOCIATE EDITORS MORRIS L. POVAR, CONSULTING EDITOR ELVA MATHIESEN, ASSISTANT EDITOR ALLAN M. SCHRIER, FOUNDING EDITOR, 1962-1987 Published Quarterly by the Schrier Research Laboratory Psychology Department, Brown University Providence, Rhode Island ISSN 0023-6861 POLICY STATEMENT The Laboratory Primate Newsletter provides a central source of information about nonhuman primates and re- lated matters to scientists who use these animals in their research and those whose work supports such research. The Newsletter (1) provides information on care and breeding of nonhuman primates for laboratory research, (2) dis- seminates general information and news about the world of primate research (such as announcements of meetings, research projects, sources of information, nomenclature changes), (3) helps meet the special research needs of indi- vidual investigators by publishing requests for research material or for information related to specific research prob- lems, and (4) serves the cause of conservation of nonhuman primates by publishing information on that topic. As a rule, research articles or summaries accepted for the Newsletter have some practical implications or provide general information likely to be of interest to investigators in a variety of areas of primate research. However, special con- sideration will be given to articles containing data on primates not conveniently publishable elsewhere. General descriptions of current research projects on primates will also be welcome. The Newsletter appears quarterly and is intended primarily for persons doing research with nonhuman primates. Back issues may be purchased for $5.00 each. -

71St Annual Meeting Society of Vertebrate Paleontology Paris Las Vegas Las Vegas, Nevada, USA November 2 – 5, 2011 SESSION CONCURRENT SESSION CONCURRENT
ISSN 1937-2809 online Journal of Supplement to the November 2011 Vertebrate Paleontology Vertebrate Society of Vertebrate Paleontology Society of Vertebrate 71st Annual Meeting Paleontology Society of Vertebrate Las Vegas Paris Nevada, USA Las Vegas, November 2 – 5, 2011 Program and Abstracts Society of Vertebrate Paleontology 71st Annual Meeting Program and Abstracts COMMITTEE MEETING ROOM POSTER SESSION/ CONCURRENT CONCURRENT SESSION EXHIBITS SESSION COMMITTEE MEETING ROOMS AUCTION EVENT REGISTRATION, CONCURRENT MERCHANDISE SESSION LOUNGE, EDUCATION & OUTREACH SPEAKER READY COMMITTEE MEETING POSTER SESSION ROOM ROOM SOCIETY OF VERTEBRATE PALEONTOLOGY ABSTRACTS OF PAPERS SEVENTY-FIRST ANNUAL MEETING PARIS LAS VEGAS HOTEL LAS VEGAS, NV, USA NOVEMBER 2–5, 2011 HOST COMMITTEE Stephen Rowland, Co-Chair; Aubrey Bonde, Co-Chair; Joshua Bonde; David Elliott; Lee Hall; Jerry Harris; Andrew Milner; Eric Roberts EXECUTIVE COMMITTEE Philip Currie, President; Blaire Van Valkenburgh, Past President; Catherine Forster, Vice President; Christopher Bell, Secretary; Ted Vlamis, Treasurer; Julia Clarke, Member at Large; Kristina Curry Rogers, Member at Large; Lars Werdelin, Member at Large SYMPOSIUM CONVENORS Roger B.J. Benson, Richard J. Butler, Nadia B. Fröbisch, Hans C.E. Larsson, Mark A. Loewen, Philip D. Mannion, Jim I. Mead, Eric M. Roberts, Scott D. Sampson, Eric D. Scott, Kathleen Springer PROGRAM COMMITTEE Jonathan Bloch, Co-Chair; Anjali Goswami, Co-Chair; Jason Anderson; Paul Barrett; Brian Beatty; Kerin Claeson; Kristina Curry Rogers; Ted Daeschler; David Evans; David Fox; Nadia B. Fröbisch; Christian Kammerer; Johannes Müller; Emily Rayfield; William Sanders; Bruce Shockey; Mary Silcox; Michelle Stocker; Rebecca Terry November 2011—PROGRAM AND ABSTRACTS 1 Members and Friends of the Society of Vertebrate Paleontology, The Host Committee cordially welcomes you to the 71st Annual Meeting of the Society of Vertebrate Paleontology in Las Vegas. -

Extinction Patterns, Δ18 O Trends, and Magnetostratigraphy from a Southern High-Latitude Cretaceous–Paleogene Section: Links with Deccan Volcanism
Palaeogeography, Palaeoclimatology, Palaeoecology 350–352 (2012) 180–188 Contents lists available at SciVerse ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Extinction patterns, δ18 O trends, and magnetostratigraphy from a southern high-latitude Cretaceous–Paleogene section: Links with Deccan volcanism Thomas S. Tobin a,⁎, Peter D. Ward a, Eric J. Steig a, Eduardo B. Olivero b, Isaac A. Hilburn c, Ross N. Mitchell d, Matthew R. Diamond c, Timothy D. Raub e, Joseph L. Kirschvink c a University of Washington, Earth and Space Sciences, Box 351310, Seattle WA 98195, United States b CADIC-CONICET, Bernardo Houssay, V9410CAB, Ushuaia, Tierra del Fuego, Argentina c California Institute of Technology, Geological and Planetary Sciences, 1200 E. California Blvd. Pasadena CA 91125, United States d Yale University, Geology & Geophysics, 230 Whitney Ave. New Haven, CT 06511, United States e University of St. Andrews, Department of Earth Sciences, St. Andrews KY16 9AL, UK article info abstract Article history: Although abundant evidence now exists for a massive bolide impact coincident with the Cretaceous–Paleogene Received 19 April 2012 (K–Pg) mass extinction event (~65.5 Ma), the relative importance of this impact as an extinction mechanism is Received in revised form 27 May 2012 still the subject of debate. On Seymour Island, Antarctic Peninsula, the López de Bertodano Formation yields one Accepted 8 June 2012 of the most expanded K–Pg boundary sections known. Using a new chronology from magnetostratigraphy, and Available online 10 July 2012 isotopic data from carbonate-secreting macrofauna, we present a high-resolution, high-latitude paleotemperature record spanning this time interval. -

The Evolutive Dynamic of the Bank Vole (Myodes Glareolus): Spatial
The evolutive dynamic of the bank vole (Myodes glareolus) : Spatial structure of the morphometric variations Ronan Ledevin To cite this version: Ronan Ledevin. The evolutive dynamic of the bank vole (Myodes glareolus) : Spatial structure of the morphometric variations. Paleontology. Université Claude Bernard - Lyon I, 2010. English. NNT : 2010LYO10196. tel-00832801 HAL Id: tel-00832801 https://tel.archives-ouvertes.fr/tel-00832801 Submitted on 11 Jun 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. N° d’ordre : 196 - 2010 Année 2010 THESE Présentée devant l’UNIVERSITE CLAUDE BERNARD – LYON 1 pour l’obtention du DIPLOME DE DOCTORAT (arrêté du 7 août 2006) Présentée et soutenue publiquement le 25 Octobre 2010 Par M. Ronan LEDEVIN La dynamique évolutive du campagnol roussâtre (Myodes glareolus) : structure spatiale des variations morphométriques Jury Rapporteurs : M. J.-C. AUFFRAY : Directeur de Recherche (Université de Montpellier II) M. A. CARDINI : Lecturer (Universitá di Modena e Reggio Emilia) Examinateurs : Mme D. PONTIER : Professeur des Universités (Université de Lyon I) M. J. R. MICHAUX : Chercheur Qualifié (Université de Liège, en accueil au CBGP de Montpellier) Directeur de Thèse : Mme S. RENAUD : Chargé de Recherche (Université de Lyon I) N° d’ordre : Année 2010 THESE Présentée devant l’UNIVERSITE CLAUDE BERNARD – LYON 1 pour l’obtention du DIPLOME DE DOCTORAT (arrêté du 7 août 2006) Présentée et soutenue publiquement le 25 Octobre 2010 Par M. -

Estimating Rates of Local Species Extinction, Colonization, and Turnover in Animal Communities
Ecological Applications, 8(4), 1998, pp. 1213±1225 q 1998 by the Ecological Society of America ESTIMATING RATES OF LOCAL SPECIES EXTINCTION, COLONIZATION, AND TURNOVER IN ANIMAL COMMUNITIES JAMES D. NICHOLS,1 THIERRY BOULINIER,2 JAMES E. HINES,1 KENNETH H. POLLOCK,3 AND JOHN R. SAUER1 1U.S. Geological Survey, Biological Resources Division, Patuxent Wildlife Research Center, Laurel, Maryland 20708 USA 2North Carolina Cooperative Fish and Wildlife Research Unit, North Carolina State University, Raleigh, North Carolina 27695 USA 3Institute of Statistics, North Carolina State University, Box 8203, Raleigh, North Carolina 27695-8203 USA Abstract. Species richness has been identi®ed as a useful state variable for conservation and management purposes. Changes in richness over time provide a basis for predicting and evaluating community responses to management, to natural disturbance, and to changes in factors such as community composition (e.g., the removal of a keystone species). Prob- abilistic capture±recapture models have been used recently to estimate species richness from species count and presence±absence data. These models do not require the common assumption that all species are detected in sampling efforts. We extend this approach to the development of estimators useful for studying the vital rates responsible for changes in animal communities over time: rates of local species extinction, turnover, and coloni- zation. Our approach to estimation is based on capture±recapture models for closed animal populations that permit heterogeneity in detection probabilities among the different species in the sampled community. We have developed a computer program, COMDYN, to compute many of these estimators and associated bootstrap variances. -

Addressing the Mammoth in the Room: the Ethical and Political Implications of De- Extinction
Addressing the Mammoth in the room: The ethical and political implications of de- extinction (Ashlock, 2013) Lowieke Vermeulen (S4374452) Political Science: Political Theory Radboud University, Nijmegen, Netherlands Supervisor: prof. dr. Marcel Wissenburg Date: August 12, 2019 Word count: 23590 1 Table of Contents Chapter 1: Introduction...............................................................................................................3 1.2 Thesis structure............................................................................................................................6 Chapter 2: De-extinction and species selection..........................................................8 2.1 Extinction........................................................................................................................................9 2.2 Approaches to de-extinction.................................................................................................10 2.2.1 Back-breeding.........................................................................................................................10 2.2.2 Cloning.......................................................................................................................................12 2.2.3 Genetic engineering..............................................................................................................12 2.2.4 Mixed approaches..................................................................................................................13 2.3 -

Punctuated Equilibrium Vs. Phyletic Gradualism
International Journal of Bio-Science and Bio-Technology Vol. 3, No. 4, December, 2011 Punctuated Equilibrium vs. Phyletic Gradualism Monalie C. Saylo1, Cheryl C. Escoton1 and Micah M. Saylo2 1 University of Antique, Sibalom, Antique, Philippines 2 DepEd Sibalom North District, Sibalom, Antique, Philippines [email protected] Abstract Both phyletic gradualism and punctuated equilibrium are speciation theory and are valid models for understanding macroevolution. Both theories describe the rates of speciation. For Gradualism, changes in species is slow and gradual, occurring in small periodic changes in the gene pool, whereas for Punctuated Equilibrium, evolution occurs in spurts of relatively rapid change with long periods of non-change. The gradualism model depicts evolution as a slow steady process in which organisms change and develop slowly over time. In contrast, the punctuated equilibrium model depicts evolution as long periods of no evolutionary change followed by rapid periods of change. Both are models for describing successive evolutionary changes due to the mechanisms of evolution in a time frame. Keywords: macroevolution, phyletic gradualism, punctuated equilibrium, speciation, evolutionary change 1. Introduction Has the evolution of life proceeded as a gradual stepwise process, or through relatively long periods of stasis punctuated by short periods of rapid evolution? To date, what is clear is that both evolutionary patterns – phyletic gradualism and punctuated equilibrium have played at least some role in the evolution of life. Gradualism and punctuated equilibrium are two ways in which the evolution of a species can occur. A species can evolve by only one of these, or by both. Scientists think that species with a shorter evolution evolved mostly by punctuated equilibrium, and those with a longer evolution evolved mostly by gradualism. -
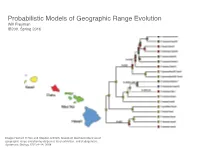
Probabilistic Models of Geographic Range Evolution Will Freyman 10 SYSTEMATIC BIOLOGY VOL
Probabilistic Models of Geographic Range Evolution Will Freyman 10 SYSTEMATIC BIOLOGY VOL. 57 IB200, Spring 2016 Downloaded from http://sysbio.oxfordjournals.org/ at University of California School Law (Boalt Hall) on April 12, 2016 Image: Richard H Ree and Stephen A Smith. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology, 57(1):4–14, 2008. FIGURE 3. Copyedited by: TRJ MANUSCRIPT CATEGORY: Article 2013 LANDIS ET AL.—BAYESIAN BIOGEOGRAPHY FOR MANY AREAS 3 A) B) There are an infinite number of biogeographic 1234011001 010 011 histories that can explain the observed geographic 011 001 ranges. When calculating the probability of the observed Biogeographic histories on geographic ranges at the tips of the phylogenetic tree, 010 a phylogeny: 5 011 it is unreasonable to condition on a specific history 001 101 of biogeographic change. After all, the past history 101 of biogeographic change is not observable. Instead, 6 111 011 the usual approach is to marginalize over all possible 111 histories of biogeographic change that could give rise 7 to the observed geographic ranges. The standard way 111 Downloaded from 101 to do this is to assume that events of colonization or local extinction occur according to a continuous- 8 101 time Markov chain (Ree et al. 2005). Marginalizing over histories of biogeographic change is accomplished C) D) 011101 010 011 011001 010 011 using two procedures. First, exponentiation of the 010 instantaneous-rate matrix, Q, gives the probability http://sysbio.oxfordjournals.org/ 011 010 011 011 011 011 111 111 001 density of all possible biogeographic changes along a 011 010 branch 111 010 101 011 011 111 011 001 Qt 111 101 011 p(y z t,Q) e− , 111 101 → ; = yz 001 % & 101 111 111 011 where y is the ancestral geographic range, z is the 011 001 current geographic range, and t is the duration of the 001 011 branch on the tree. -

Rainer Ulrich 2002.Pdf
New Developments in Viral Vaccine Technologies Guest Editors Rainer Ulrich, Berlin George P. Lomonossoff, Norwich Detlev H. Krüger, Berlin 73 figures and 39 tables, 2002 Basel Ⴇ Freiburg Ⴇ Paris Ⴇ London Ⴇ New York Ⴇ Bangalore Ⴇ Bangkok Ⴇ Singapore Ⴇ Tokyo Ⴇ Sydney S. Karger Drug Dosage All rights reserved. Medical and Scientific Publishers The authors and the publisher have exerted every effort to en- No part of this publication may be translated into other Basel Ⴇ Freiburg Ⴇ Paris Ⴇ London sure that drug selection and dosage set forth in this text are in languages, reproduced or utilized in any form or by any means, Ⴇ Ⴇ accord with current recommendations and practice at the time electronic or mechanical, including photocopying, recording, New York Bangalore Bangkok of publication. However, in view of ongoing research, changes microcopying, or by any information storage and retrieval Ⴇ Ⴇ Singapore Tokyo Sydney in government regulations, and the constant flow of informa- system, without permission in writing from the publisher or, in tion relating to drug therapy and drug reactions, the reader is the case of photocopying, direct payment of a specified fee to urged to check the package insert for each drug for any change the Copyright Clearance Center (see ‘General Information’). in indications and dosage and for added warnings and precau- tions. This is particularly important when the recommended © Copyright 2002 by S. Karger AG, agent is a new and/or infrequently employed drug. P.O. Box, CH–4009 Basel (Switzerland) Printed in Switzerland on acid-free paper by Reinhardt Druck, Basel ISBN 3–8055–7505–X Fax + 41 61 306 12 34 E-Mail [email protected] www.karger.com Contents Vol. -

New Information on the Late Pleistocene Birds from San Josecito Cave, Nuevo Leon, Mexico ’
A JOURNAL OF AVIAN BIOLOGY Volume 96 Number 3 The Condor96571-589 Q The Cooper Omithologkzd %cietY 1994 NEW INFORMATION ON THE LATE PLEISTOCENE BIRDS FROM SAN JOSECITO CAVE, NUEVO LEON, MEXICO ’ DAVID W. STEADMAN New York State Museum, The State Education Department, Albany, NY 12230 JOAQUIN ARROYO-CARRALES Museum of Texas Tech University,Lubbock, TX 79409 and Laboratorio de Paleozoologia,Subdireccion de ServiciosAcademicos, Instituto National de Antropologiae Historia, Mexico EILEEN JOHNSON Museum of Texas Tech University,Lubbock, TX 79409 A. FARIOLA GUZMAN Laboratorio de Paleozoologta,Subdireccibn de ServiciosAcademicos, Instituto National de Antropologiiae Historia, Mexico Abstract. We report 90 bird bones representing 18 speciesfrom recent excavations at San Josecito Gave, Nuevo Le6n, Mexico. The new material increasesthe avifauna of this rich late Pleistocenelocality from 52 to 62 species.Eight of the 10 newly recorded taxa are extant; each is either of temperate rather than tropical affinities (such as the American Woodcock Scolopax minor and Pinyon Jay Gymnorhinuscyanocephalus) or is very wide- spreadin its modem distribution. The two extinct taxa are a stork (Ciconia sp. or Mycteria sp.) and Geococcyxcalifornianus conklingi, a large temporal subspeciesof the Greater Road- runner. In this region of the Sierra Madre Oriental (about lat. 24”N, long. lOO”W, elev. 2,000-2,600 m). the late Pleistocene avifauna was a mixture of speciesthat to&y prefer coniferous or pine-oak forests/woodlands,grasslands/savannas, and wetlands. As with var- ious late Pleistoceneplant and mammal communities of the United Statesand Mexico, no clear modem analog exists for the late Pleistoceneavifauna of San JosecitoCave. Key words: Late Pleistoceneavzfaunas; Mexico; historicalbiogeography; extinct species; temperate/tropicaltransition. -

Determining Species Expansion and Extinction Possibilities Using Probabilistic and Graphical Models
Ekológia (Bratislava) Vol. 34, No. 2, p. 111–120, 2015 DOI:10.1515/eko-2015-0012 DetermininG species EXpansion AND EXtinction possibilities UsinG probabilistic AND Graphical models RAJESH CHATURVEDI, P. RAMA CHANDRA PRASAD Lab for Spatial Informatics, International Institute of Information Technology, Gachibowli, Hyderabad - 500032, India; e-mail: [email protected], [email protected] Abstract Chaturvedi R., Prasad R.Ch.: Determining species expansion and extinction possibilities using probabilistic and graphical models. Ekológia (Bratislava), Vol. 34, No. 2, p. 111–120, 2015. Survival of plant species is governed by a number of functions. The participation of each function in species survival and the impact of the contrary behaviour of the species vary from function to function. The probability of extinction of species varies in all such scenarios and has to be calculated separately. Secondly, species follow different patterns of dispersal and localisation at different stages of occupancy state of the site, therefore, the scenarios of competition for resources with climatic shifts leading to deterioration and loss of biodiversity resulting in extinction needs to be studied. Furthermore, most po- ssible deviations of species from climax community states needs to be calculated before species become extinct due to sudden environmental disruption. Globally, various types of anthropogenic disturbances threaten the diversity of biological systems. The impact of these anthropogenic activities needs to be analysed to identify extinction patterns with respect to these activities. All the analyses mentioned abo- ve have been tried to be achieved through probabilistic or graphical models in this study. Key words: biodiversity, disturbances, climate change, endangered, risks, ecological models. -

Mammalian Evolution During the Cretaceous-Tertiary Transition; Evidence Fo~
Acta Palaeontologica Polonica Vol. 28, No. 1-2 pp, 55-61 Warszawa, 1983 Second Symposium on Mesozoic T errestial Ecosystems, Jadwisin 1981 WILLIAM A. CLEMENS MAMMALIAN EVOLUTION DURING THE CRETACEOUS-TERTIARY TRANSITION; EVIDENCE FO~ . GRADUAL, NON-CATASTROPHIC PATTERNS OF BIOTIC CHANGE CLEMENS WILLIAM A. : Mammalian evolution during the Cretaceous-Tertiary transition; ev idence for gradual, non-catastrophic patterns of biotic change. Acta Palaeont. Polonlca, 28, 1-2, 55-61, 1983, Hypotheses Invoking catastrophic causal factors for terminal Cretaceous extinctions call for, 1) Instantaneous, global extinctions, 2) resulting from a single or dominant causal factor. 'c u rr entl y available methods of correlation are not precise enough to test the first element of these hypotheses. Patterns of mammalian extinction and survival In northeastern Montana, USA - as well as records of other organisms - argue against the second element. In Montana most of the lineages of marsupials were decimated. Extinction of multltuber curates was not as severe, while only one lineage of eutherlans appears to have become extinct. Groups that apparently Immigrated Into northeastern Montana just prior to the end of the Cretaceous had a greater probability of survival than the residents. The prObability of extinction was Independent of Individual body size. Different patterns of mammalian extinction In different environments also appear to defy explanation In terms of one common, catastrophic causal factor. Key w 0 r d s: Mammalia, Cretaceous, Tertiary, extinction, Montana, USA . Wltltam A . Clemens. Department Of Paleontology. University 01 Callfornla. Berkeley, Callfornla. 94720. USA. Recei ved: September 1981. INTRODUCTION Scientific and popular publications continue to be flooded with articles concerning the causes of the extinction of dinosaurs and other organisms used to mark the end of the Cretaceous.