Determining Species Expansion and Extinction Possibilities Using Probabilistic and Graphical Models
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SDG Indicator Metadata (Harmonized Metadata Template - Format Version 1.0)
Last updated: 4 January 2021 SDG indicator metadata (Harmonized metadata template - format version 1.0) 0. Indicator information 0.a. Goal Goal 15: Protect, restore and promote sustainable use of terrestrial ecosystems, sustainably manage forests, combat desertification, and halt and reverse land degradation and halt biodiversity loss 0.b. Target Target 15.5: Take urgent and significant action to reduce the degradation of natural habitats, halt the loss of biodiversity and, by 2020, protect and prevent the extinction of threatened species 0.c. Indicator Indicator 15.5.1: Red List Index 0.d. Series 0.e. Metadata update 4 January 2021 0.f. Related indicators Disaggregations of the Red List Index are also of particular relevance as indicators towards the following SDG targets (Brooks et al. 2015): SDG 2.4 Red List Index (species used for food and medicine); SDG 2.5 Red List Index (wild relatives and local breeds); SDG 12.2 Red List Index (impacts of utilisation) (Butchart 2008); SDG 12.4 Red List Index (impacts of pollution); SDG 13.1 Red List Index (impacts of climate change); SDG 14.1 Red List Index (impacts of pollution on marine species); SDG 14.2 Red List Index (marine species); SDG 14.3 Red List Index (reef-building coral species) (Carpenter et al. 2008); SDG 14.4 Red List Index (impacts of utilisation on marine species); SDG 15.1 Red List Index (terrestrial & freshwater species); SDG 15.2 Red List Index (forest-specialist species); SDG 15.4 Red List Index (mountain species); SDG 15.7 Red List Index (impacts of utilisation) (Butchart 2008); and SDG 15.8 Red List Index (impacts of invasive alien species) (Butchart 2008, McGeoch et al. -

Improvements to the Red List Index Stuart H
Improvements to the Red List Index Stuart H. M. Butchart1*, H. Resit Akc¸akaya2, Janice Chanson3, Jonathan E. M. Baillie4, Ben Collen4, Suhel Quader5,8, Will R. Turner6, Rajan Amin4, Simon N. Stuart3, Craig Hilton-Taylor7 1 BirdLife International, Cambridge, United Kingdom, 2 Applied Biomathematics, Setauket, New York, United States of America, 3 Conservation International/Center for Applied Biodiversity Science-World Conservation Union (IUCN)/Species Survival Commission Biodiversity Assessment Unit, IUCN Species Programme, Center for Applied Biodiversity Science, Conservation International, Washington, D. C., United States of America, 4 Institute of Zoology, Zoological Society of London, London, United Kingdom, 5 Department of Zoology, University of Cambridge, Cambridge, United Kingdom, 6 Center for Applied Biodiversity Science, Conservation International, Washington, D. C., United States of America, 7 World Conservation Union (IUCN) Species Programme, Cambridge, United Kingdom, 8 Royal Society for the Protection of Birds, Sandy, United Kingdom The Red List Index uses information from the IUCN Red List to track trends in the projected overall extinction risk of sets of species. It has been widely recognised as an important component of the suite of indicators needed to measure progress towards the international target of significantly reducing the rate of biodiversity loss by 2010. However, further application of the RLI (to non-avian taxa in particular) has revealed some shortcomings in the original formula and approach: It performs inappropriately when a value of zero is reached; RLI values are affected by the frequency of assessments; and newly evaluated species may introduce bias. Here we propose a revision to the formula, and recommend how it should be applied in order to overcome these shortcomings. -

A Robust Goal Is Needed for Species in the Post-2020 Global Biodiversity Framework
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 16 April 2020 Title: A robust goal is needed for species in the Post-2020 Global Biodiversity Framework Running title: A post-2020 goal for species conservation Authors: Brooke A. Williams*1,2, James E.M. Watson1,2,3, Stuart H.M. Butchart4,5, Michelle Ward1,2, Nathalie Butt2, Friederike C. Bolam6, Simon N. Stuart7, Thomas M. Brooks8,9,10, Louise Mair6, Philip J. K. McGowan6, Craig Hilton-Taylor11, David Mallon12, Ian Harrison8,13, Jeremy S. Simmonds1,2. Affiliations: 1School of Earth and Environmental Sciences, University of Queensland, St Lucia 4072, Australia 2Centre for Biodiversity and Conservation Science, School of Biological Sciences, University of Queensland, St Lucia 4072, Queensland, Australia. 3Wildlife Conservation Society, Global Conservation Program, Bronx, NY 20460, USA. 4BirdLife International, The David Attenborough Building, Pembroke Street, Cambridge CB2 3QZ, UK 5Department of Zoology, Cambridge University, Downing Street, Cambridge CB2 3EJ, UK 6School of Natural and Environmental Sciences, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK 7Synchronicity Earth, 27-29 Cursitor Street, London, EC4A 1LT, UK 8IUCN, 28 rue Mauverney, CH-1196, Gland, Switzerland 9World Agroforestry Center (ICRAF), University of the Philippines Los Baños, Laguna, 4031, Philippines 10Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, TAS 7001, Australia 11IUCN, The David Attenborough Building, Pembroke Street, Cambridge CB2 3QZ, UK 12Division of Biology and Conservation Ecology, Manchester Metropolitan University, Chester St, Manchester, M1 5GD, U.K 13Conservation International, Arlington, VA 22202, USA Emails: Brooke A. Williams: [email protected]; James E.M. Watson: [email protected]; Stuart H.M. -

Extinction Patterns, Δ18 O Trends, and Magnetostratigraphy from a Southern High-Latitude Cretaceous–Paleogene Section: Links with Deccan Volcanism
Palaeogeography, Palaeoclimatology, Palaeoecology 350–352 (2012) 180–188 Contents lists available at SciVerse ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Extinction patterns, δ18 O trends, and magnetostratigraphy from a southern high-latitude Cretaceous–Paleogene section: Links with Deccan volcanism Thomas S. Tobin a,⁎, Peter D. Ward a, Eric J. Steig a, Eduardo B. Olivero b, Isaac A. Hilburn c, Ross N. Mitchell d, Matthew R. Diamond c, Timothy D. Raub e, Joseph L. Kirschvink c a University of Washington, Earth and Space Sciences, Box 351310, Seattle WA 98195, United States b CADIC-CONICET, Bernardo Houssay, V9410CAB, Ushuaia, Tierra del Fuego, Argentina c California Institute of Technology, Geological and Planetary Sciences, 1200 E. California Blvd. Pasadena CA 91125, United States d Yale University, Geology & Geophysics, 230 Whitney Ave. New Haven, CT 06511, United States e University of St. Andrews, Department of Earth Sciences, St. Andrews KY16 9AL, UK article info abstract Article history: Although abundant evidence now exists for a massive bolide impact coincident with the Cretaceous–Paleogene Received 19 April 2012 (K–Pg) mass extinction event (~65.5 Ma), the relative importance of this impact as an extinction mechanism is Received in revised form 27 May 2012 still the subject of debate. On Seymour Island, Antarctic Peninsula, the López de Bertodano Formation yields one Accepted 8 June 2012 of the most expanded K–Pg boundary sections known. Using a new chronology from magnetostratigraphy, and Available online 10 July 2012 isotopic data from carbonate-secreting macrofauna, we present a high-resolution, high-latitude paleotemperature record spanning this time interval. -

1 Chapter 1.6. Evolution While You Are Watching Chapter 1.6
Chapter 1.6. Evolution While You Are Watching Chapter 1.6 describes more or less direct observations of evolution. Although Macroevolution which results in really profound changes of living beings occurs too slowly for such observations, substantial changes in a lineage can and do occur at the time scale of thousands or even hundreds of generations. Fast evolution is usually driven by strong selection and can involve numerous allele replacements. Direct observations of evolution come from data of four kinds. Section 1.6.1 treats observations of rapid evolution of nature. Here we will understand the concept of observation broadly, and include data on changes of phenotypes in continuous paleontological records and on local adaptation. However, under some circumstances evolution proceeds so rapidly that substantial changes occur in the course of decades, and, thus, can be literally observed. Section 1.6.2 considers domestication, a phenomenon which became common after the origin agriculture ~12,000 years ago, and evolution of domesticated plants and animals. Due to artificial selection and to natural selection under new environment, important phenotypes of many domesticated species are currently way outside the range of their natural variation, and the diversity of varieties is astonishing. Thus, domesticated species provide a rich source of data on evolution at a relatively small scale. Section 1.6.3 reviews data on experimental evolution in controlled populations of a variety of organisms. Such experiments are an important tool of evolutionary biology, which made it possible to directly observe a number of key phenomena. However, a limitation of evolutionary experiments is that they must deal with organisms with short generation times. -

Estimating Rates of Local Species Extinction, Colonization, and Turnover in Animal Communities
Ecological Applications, 8(4), 1998, pp. 1213±1225 q 1998 by the Ecological Society of America ESTIMATING RATES OF LOCAL SPECIES EXTINCTION, COLONIZATION, AND TURNOVER IN ANIMAL COMMUNITIES JAMES D. NICHOLS,1 THIERRY BOULINIER,2 JAMES E. HINES,1 KENNETH H. POLLOCK,3 AND JOHN R. SAUER1 1U.S. Geological Survey, Biological Resources Division, Patuxent Wildlife Research Center, Laurel, Maryland 20708 USA 2North Carolina Cooperative Fish and Wildlife Research Unit, North Carolina State University, Raleigh, North Carolina 27695 USA 3Institute of Statistics, North Carolina State University, Box 8203, Raleigh, North Carolina 27695-8203 USA Abstract. Species richness has been identi®ed as a useful state variable for conservation and management purposes. Changes in richness over time provide a basis for predicting and evaluating community responses to management, to natural disturbance, and to changes in factors such as community composition (e.g., the removal of a keystone species). Prob- abilistic capture±recapture models have been used recently to estimate species richness from species count and presence±absence data. These models do not require the common assumption that all species are detected in sampling efforts. We extend this approach to the development of estimators useful for studying the vital rates responsible for changes in animal communities over time: rates of local species extinction, turnover, and coloni- zation. Our approach to estimation is based on capture±recapture models for closed animal populations that permit heterogeneity in detection probabilities among the different species in the sampled community. We have developed a computer program, COMDYN, to compute many of these estimators and associated bootstrap variances. -

Addressing the Mammoth in the Room: the Ethical and Political Implications of De- Extinction
Addressing the Mammoth in the room: The ethical and political implications of de- extinction (Ashlock, 2013) Lowieke Vermeulen (S4374452) Political Science: Political Theory Radboud University, Nijmegen, Netherlands Supervisor: prof. dr. Marcel Wissenburg Date: August 12, 2019 Word count: 23590 1 Table of Contents Chapter 1: Introduction...............................................................................................................3 1.2 Thesis structure............................................................................................................................6 Chapter 2: De-extinction and species selection..........................................................8 2.1 Extinction........................................................................................................................................9 2.2 Approaches to de-extinction.................................................................................................10 2.2.1 Back-breeding.........................................................................................................................10 2.2.2 Cloning.......................................................................................................................................12 2.2.3 Genetic engineering..............................................................................................................12 2.2.4 Mixed approaches..................................................................................................................13 2.3 -

Global Conservation Translocation Perspectives: 2021. Case Studies from Around the Globe
Global conservation Global conservation translocation perspectives: 2021 translocation perspectives: 2021 IUCN SSC Conservation Translocation Specialist Group Global conservation translocation perspectives: 2021 Case studies from around the globe Edited by Pritpal S. Soorae IUCN SSC Conservation Translocation Specialist Group (CTSG) i The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN or any of the funding organizations concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN. IUCN is pleased to acknowledge the support of its Framework Partners who provide core funding: Ministry of Foreign Affairs of Denmark; Ministry for Foreign Affairs of Finland; Government of France and the French Development Agency (AFD); the Ministry of Environment, Republic of Korea; the Norwegian Agency for Development Cooperation (Norad); the Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC) and the United States Department of State. Published by: IUCN SSC Conservation Translocation Specialist Group, Environment Agency - Abu Dhabi & Calgary Zoo, Canada. Copyright: © 2021 IUCN, International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non- commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Soorae, P. S. -
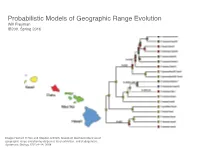
Probabilistic Models of Geographic Range Evolution Will Freyman 10 SYSTEMATIC BIOLOGY VOL
Probabilistic Models of Geographic Range Evolution Will Freyman 10 SYSTEMATIC BIOLOGY VOL. 57 IB200, Spring 2016 Downloaded from http://sysbio.oxfordjournals.org/ at University of California School Law (Boalt Hall) on April 12, 2016 Image: Richard H Ree and Stephen A Smith. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology, 57(1):4–14, 2008. FIGURE 3. Copyedited by: TRJ MANUSCRIPT CATEGORY: Article 2013 LANDIS ET AL.—BAYESIAN BIOGEOGRAPHY FOR MANY AREAS 3 A) B) There are an infinite number of biogeographic 1234011001 010 011 histories that can explain the observed geographic 011 001 ranges. When calculating the probability of the observed Biogeographic histories on geographic ranges at the tips of the phylogenetic tree, 010 a phylogeny: 5 011 it is unreasonable to condition on a specific history 001 101 of biogeographic change. After all, the past history 101 of biogeographic change is not observable. Instead, 6 111 011 the usual approach is to marginalize over all possible 111 histories of biogeographic change that could give rise 7 to the observed geographic ranges. The standard way 111 Downloaded from 101 to do this is to assume that events of colonization or local extinction occur according to a continuous- 8 101 time Markov chain (Ree et al. 2005). Marginalizing over histories of biogeographic change is accomplished C) D) 011101 010 011 011001 010 011 using two procedures. First, exponentiation of the 010 instantaneous-rate matrix, Q, gives the probability http://sysbio.oxfordjournals.org/ 011 010 011 011 011 011 111 111 001 density of all possible biogeographic changes along a 011 010 branch 111 010 101 011 011 111 011 001 Qt 111 101 011 p(y z t,Q) e− , 111 101 → ; = yz 001 % & 101 111 111 011 where y is the ancestral geographic range, z is the 011 001 current geographic range, and t is the duration of the 001 011 branch on the tree. -

A Process for Assessing and Prioritzing Species Conservaton Needs: Going Beyond the Red List
A process for assessing and prioritzing species conservaton needs: going beyond the red list KEVIN JOHNSON, ANNE BAKER, KEVIN BULEY, LUIS CARRILLO, RICHARD GIBSON, GRAEME GILLESPIE, ROBERT C. LACY and KEVIN ZIPPEL SUPPLEMENTARY MATERIAL 1 Assessment Questions and Answer Scores. Assessment questions are designed to serve two purposes: to identify the needed conservation actions for each species and for quantitative prioritization of species for each action. Numeric scores from questions are used to develop the overall prioritization score, with the scores for the selected responses added to give a total. A higher total score represents a species of higher priority. Questions without scores are used as triggers for conservation actions, or to provide additional information to support subsequent action-planning, but are not used in the prioritization (scoring) process. Assessors select the most appropriate response to each question for the species being assessed. Section One – Review of external data 1. Extinction risk: What is the current IUCN Red List category for the taxon? The Red List category can be modified accordingly (for the purposes of this assessment only) if new/additional information is available, or if country-level Red List assessments exist. If the assessors consider that the Red List category of threat would change if the species was re- assessed using more current data than that which was used previously, or if a more recent national Red List assessment exists, a revised estimate of the new category can be chosen, and this will be used to calculate priorities and conservation actions. If a national Red List assessment exists, the national category of threat is used rather than the global category. -

Mexico's Ten Most Iconic Endangered Species
Alejandro Olivera Center for Biological Diversity, April 2018 Executive summary exico is one of the world’s most biologically rich nations, with diverse landscapes that are home to a treasure trove of wildlife, including plant and animal species found nowhere else. Sadly, in Mexico and Maround the world, species are becoming extinct because of human activities at rates never seen before. In this report we highlight the threats facing Mexico’s 10 most iconic endangered species to help illustrate the broader risks confronting the country’s imperiled plants and animals. These 10 species — which in most cases are protected only on paper — were chosen to reflect Mexico’s diversity of wildlife and ecosystems and the wide range of threats to the country’s biodiversity. New awareness of these unique animals and plants is critical to inspiring a nationwide demand to protect these critical components of Mexico’s natural heritage. Although the Mexican government began officially listing and protecting species as extinct, threatened, endangered, and “under special protection” in 1994 — more than 20 years ago — few species have actually recovered, and many critical threats continue unabated. In many cases, officials are failing to enforce crucial laws and regulations that would protect these species. Additionally, the Mexican government has not updated its official list of imperiled species, referred to as NOM059, since 2010, despite new and growing risks from climate change, habitat destruction, the wildlife trade and in some cases direct killing. This failure obscures the true plight of the nation’s endangered wildlife. The following 10 iconic endangered species are not adequately protected by the Mexican government: 1. -

WILDLIFE in a CHANGING WORLD an Analysis of the 2008 IUCN Red List of Threatened Species™
WILDLIFE IN A CHANGING WORLD An analysis of the 2008 IUCN Red List of Threatened Species™ Edited by Jean-Christophe Vié, Craig Hilton-Taylor and Simon N. Stuart coberta.indd 1 07/07/2009 9:02:47 WILDLIFE IN A CHANGING WORLD An analysis of the 2008 IUCN Red List of Threatened Species™ first_pages.indd I 13/07/2009 11:27:01 first_pages.indd II 13/07/2009 11:27:07 WILDLIFE IN A CHANGING WORLD An analysis of the 2008 IUCN Red List of Threatened Species™ Edited by Jean-Christophe Vié, Craig Hilton-Taylor and Simon N. Stuart first_pages.indd III 13/07/2009 11:27:07 The designation of geographical entities in this book, and the presentation of the material, do not imply the expressions of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily refl ect those of IUCN. This publication has been made possible in part by funding from the French Ministry of Foreign and European Affairs. Published by: IUCN, Gland, Switzerland Red List logo: © 2008 Copyright: © 2009 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Vié, J.-C., Hilton-Taylor, C.