Clarias Batrachus)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Supplemental Information to Mammadova-Bach Et Al., “Laminin Α1 Orchestrates VEGFA Functions in the Ecosystem of Colorectal Carcinogenesis”
Supplemental information to Mammadova-Bach et al., “Laminin α1 orchestrates VEGFA functions in the ecosystem of colorectal carcinogenesis” Supplemental material and methods Cloning of the villin-LMα1 vector The plasmid pBS-villin-promoter containing the 3.5 Kb of the murine villin promoter, the first non coding exon, 5.5 kb of the first intron and 15 nucleotides of the second villin exon, was generated by S. Robine (Institut Curie, Paris, France). The EcoRI site in the multi cloning site was destroyed by fill in ligation with T4 polymerase according to the manufacturer`s instructions (New England Biolabs, Ozyme, Saint Quentin en Yvelines, France). Site directed mutagenesis (GeneEditor in vitro Site-Directed Mutagenesis system, Promega, Charbonnières-les-Bains, France) was then used to introduce a BsiWI site before the start codon of the villin coding sequence using the 5’ phosphorylated primer: 5’CCTTCTCCTCTAGGCTCGCGTACGATGACGTCGGACTTGCGG3’. A double strand annealed oligonucleotide, 5’GGCCGGACGCGTGAATTCGTCGACGC3’ and 5’GGCCGCGTCGACGAATTCACGC GTCC3’ containing restriction site for MluI, EcoRI and SalI were inserted in the NotI site (present in the multi cloning site), generating the plasmid pBS-villin-promoter-MES. The SV40 polyA region of the pEGFP plasmid (Clontech, Ozyme, Saint Quentin Yvelines, France) was amplified by PCR using primers 5’GGCGCCTCTAGATCATAATCAGCCATA3’ and 5’GGCGCCCTTAAGATACATTGATGAGTT3’ before subcloning into the pGEMTeasy vector (Promega, Charbonnières-les-Bains, France). After EcoRI digestion, the SV40 polyA fragment was purified with the NucleoSpin Extract II kit (Machery-Nagel, Hoerdt, France) and then subcloned into the EcoRI site of the plasmid pBS-villin-promoter-MES. Site directed mutagenesis was used to introduce a BsiWI site (5’ phosphorylated AGCGCAGGGAGCGGCGGCCGTACGATGCGCGGCAGCGGCACG3’) before the initiation codon and a MluI site (5’ phosphorylated 1 CCCGGGCCTGAGCCCTAAACGCGTGCCAGCCTCTGCCCTTGG3’) after the stop codon in the full length cDNA coding for the mouse LMα1 in the pCIS vector (kindly provided by P. -

Differential Expression of Hydroxyurea Transporters in Normal and Polycythemia Vera Hematopoietic Stem and Progenitor Cell Subpopulations
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2021 Differential expression of hydroxyurea transporters in normal and polycythemia vera hematopoietic stem and progenitor cell subpopulations Tan, Ge ; Meier-Abt, Fabienne Abstract: Polycythemia vera (PV) is a myeloproliferative neoplasm marked by hyperproliferation of the myeloid lineages and the presence of an activating JAK2 mutation. Hydroxyurea (HU) is a standard treat- ment for high-risk patients with PV. Because disease-driving mechanisms are thought to arise in PV stem cells, effective treatments should target primarily the stem cell compartment. We tested for theantipro- liferative effect of patient treatment with HU in fluorescence-activated cell sorting-isolated hematopoietic stem/multipotent progenitor cells (HSC/MPPs) and more committed erythroid progenitors (common myeloid/megakaryocyte-erythrocyte progenitors [CMP/MEPs]) in PV using RNA-sequencing and gene set enrichment analysis. HU treatment led to significant downregulation of gene sets associated with cell proliferation in PV HSCs/MPPs, but not in PV CMP/MEPs. To explore the mechanism underlying this finding, we assessed for expression of solute carrier membrane transporters, which mediate trans- membrane movement of drugs such as HU into target cells. The active HU uptake transporter OCTN1 was upregulated in HSC/MPPs compared with CMP/MEPs of untreated patients with PV, and the HU diffusion facilitator urea transporter B (UTB) was downregulated in HSC/MPPs compared withCM- P/MEPs in all patient and control groups tested. These findings indicate a higher accumulation ofHU within PV HSC/MPPs compared with PV CMP/MEPs and provide an explanation for the differential effects of HU in HSC/MPPs and CMP/MEPs of patients with PV. -

Amphibious Fishes: Terrestrial Locomotion, Performance, Orientation, and Behaviors from an Applied Perspective by Noah R
AMPHIBIOUS FISHES: TERRESTRIAL LOCOMOTION, PERFORMANCE, ORIENTATION, AND BEHAVIORS FROM AN APPLIED PERSPECTIVE BY NOAH R. BRESSMAN A Dissertation Submitted to the Graduate Faculty of WAKE FOREST UNIVESITY GRADUATE SCHOOL OF ARTS AND SCIENCES in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY Biology May 2020 Winston-Salem, North Carolina Approved By: Miriam A. Ashley-Ross, Ph.D., Advisor Alice C. Gibb, Ph.D., Chair T. Michael Anderson, Ph.D. Bill Conner, Ph.D. Glen Mars, Ph.D. ACKNOWLEDGEMENTS I would like to thank my adviser Dr. Miriam Ashley-Ross for mentoring me and providing all of her support throughout my doctoral program. I would also like to thank the rest of my committee – Drs. T. Michael Anderson, Glen Marrs, Alice Gibb, and Bill Conner – for teaching me new skills and supporting me along the way. My dissertation research would not have been possible without the help of my collaborators, Drs. Jeff Hill, Joe Love, and Ben Perlman. Additionally, I am very appreciative of the many undergraduate and high school students who helped me collect and analyze data – Mark Simms, Tyler King, Caroline Horne, John Crumpler, John S. Gallen, Emily Lovern, Samir Lalani, Rob Sheppard, Cal Morrison, Imoh Udoh, Harrison McCamy, Laura Miron, and Amaya Pitts. I would like to thank my fellow graduate student labmates – Francesca Giammona, Dan O’Donnell, MC Regan, and Christine Vega – for their support and helping me flesh out ideas. I am appreciative of Dr. Ryan Earley, Dr. Bruce Turner, Allison Durland Donahou, Mary Groves, Tim Groves, Maryland Department of Natural Resources, UF Tropical Aquaculture Lab for providing fish, animal care, and lab space throughout my doctoral research. -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Abrothrix Olivacea)
Received: 13 June 2017 | Revised: 30 May 2018 | Accepted: 31 May 2018 DOI: 10.1111/mec.14778 ORIGINAL ARTICLE An association between differential expression and genetic divergence in the Patagonian olive mouse (Abrothrix olivacea) Facundo M. Giorello1,2 | Matias Feijoo1 | Guillermo D'Elía3 | Daniel E. Naya1 | Lourdes Valdez3 | Juan C. Opazo3 | Enrique P. Lessa1 1Departamento de Ecología y Evolución, Facultad de Ciencias, Universidad de la Abstract República, Montevideo, Uruguay Recent molecular studies have found striking differences between desert‐adapted 2Espacio de Biología Vegetal del Noreste, species and model mammals regarding water conservation. In particular, aquaporin Centro Universitario de Tacuarembó, Universidad de la República, Tacuarembó, 4, a classical gene involved in water regulation of model species, is absent or not Uruguay expressed in the kidneys of desert‐adapted species. To further understand the 3Instituto de Ciencias Ambientales y Evolutivas, Universidad Austral de Chile, molecular response to water availability, we studied the Patagonian olive mouse Valdivia, Chile Abrothrix olivacea, a species with an unusually broad ecological tolerance that exhi- Correspondence bits a great urine concentration capability. The species is able to occupy both the Facundo M. Giorello, Departamento de arid Patagonian steppe and the Valdivian and Magellanic forests. We sampled 95 Ecología y Evolución, Facultad de Ciencias, Universidad de la República, Iguá 4225, olive mouse specimens from four localities (two in the steppe and two in the for- Montevideo 11400, Uruguay. ests) and analysed both phenotypic variables and transcriptomic data to investigate Email: [email protected] the response of this species to the contrasting environmental conditions. The rela- Funding information tive size of the kidney and the ratio of urine to plasma concentrations were, as Fondo Nacional de Desarrollo Científico y Tecnológico, Grant/Award Number: expected, negatively correlated with annual rainfall. -

Coping with Life on Land: Physiological, Biochemical, and Structural Mechanisms to Enhance Function in Amphibious Fishes
Coping with Life on Land: Physiological, Biochemical, and Structural Mechanisms to Enhance Function in Amphibious Fishes by Andy Joseph Turko A Thesis presented to The University of Guelph In partial fulfilment of requirements for the degree of Doctor of Philosophy in Integrative Biology Guelph, Ontario, Canada © Andy Joseph Turko, October 2018 ABSTRACT COPING WITH LIFE ON LAND: PHYSIOLOGICAL, BIOCHEMICAL, AND STRUCTURAL MECHANISMS TO ENHANCE FUNCTION IN AMPHIBIOUS FISHES Andy Joseph Turko Advisor: University of Guelph, 2018 Dr. Patricia A. Wright The invasion of land by fishes was one of the most dramatic transitions in the evolutionary history of vertebrates. In this thesis, I investigated how amphibious fishes cope with increased effective gravity and the inability to feed while out of water. In response to increased body weight on land (7 d), the gill skeleton of Kryptolebias marmoratus became stiffer, and I found increased abundance of many proteins typically associated with bone and cartilage growth in mammals. Conversely, there was no change in gill stiffness in the primitive ray-finned fish Polypterus senegalus after one week out of water, but after eight months the arches were significantly shorter and smaller. A similar pattern of gill reduction occurred during the tetrapod invasion of land, and my results suggest that genetic assimilation of gill plasticity could be an underlying mechanism. I also found proliferation of a gill inter-lamellar cell mass in P. senegalus out of water (7 d) that resembled gill remodelling in several other fishes, suggesting this may be an ancestral actinopterygian trait. Next, I tested the function of a calcified sheath that I discovered surrounding the gill filaments of >100 species of killifishes and some other percomorphs. -

Structure and Permeation Mechanism of a Mammalian Urea Transporter
Structure and permeation mechanism of a mammalian urea transporter Elena J. Levina,1, Yu Caoa,1, Giray Enkavib, Matthias Quickc, Yaping Pana, Emad Tajkhorshidb,2, and Ming Zhoua,2 aDepartment of Physiology and Cellular Biophysics, College of Physicians and Surgeons, Columbia University, 630 West 168th Street, New York, NY 10032; bCenter for Biophysics and Computational Biology, Department of Biochemistry, College of Medicine, and Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL 61801; and cDepartment of Psychiatry and Center for Molecular Recognition, Columbia University, 650 West 168th Street, New York, NY 10032 Edited by Christopher Miller, HHMI, Brandeis University, Waltham, MA, and approved June 1, 2012 (received for review May 3, 2012) As an adaptation to infrequent access to water, terrestrial mam- homolog (21), dvUT, which forms a trimer with a continuous mals produce urine that is hyperosmotic to plasma. To prevent membrane-spanning pore at the center of each protomer. How- osmotic diuresis by the large quantity of urea generated by protein ever, it remained unclear how similar this structure was to that catabolism, the kidney epithelia contain facilitative urea transpor- of the mammalian UTs, and the details of the permeation me- ters (UTs) that allow rapid equilibration between the urinary space chanism were unknown. To answer these questions, we solved and the hyperosmotic interstitium. Here we report the first X-ray the structure of a mammalian UT-B and investigated the permea- crystal structure of a mammalian UT, UT-B, at a resolution of 2.36 Å. tion mechanism with molecular dynamics simulations and func- UT-B is a homotrimer and each protomer contains a urea con- tional studies of UT-B mutants. -

Non-Commercial Use Only
Journal of Xenobiotics 2018; volume 8:7820 Behavioral effects of the stage, an idea accepted under the concept of neurotoxin b-N-methylamino- Developmental Origin of Health and Correspondence: Alessandra Carion, Disease (DOHaD).2 However, even if Laboratory of Evolutionary and Adaptive L-alanine on the mangrove experimental and epidemiological studies Physiology, Institute of Life, Earth and rivulus (Kryptolebias support this concept, the mechanisms Environment, University of Namur, 61 Rue de Bruxelles, 5000 Namur, Belgium. explaining long-term delayed effects of marmoratus) larvae E-mail: [email protected] early life NCs exposure remain largely unclear.3 Alessandra Carion, Julie Hétru, Key words: Mangrove rivulus; Nowadays, an increasing number of Developmental origin of health and disease; Angèle Markey, people suffer from neurological disorders Neurotoxin; Behavior; b-N-methylamino-L- Victoria Suarez-Ulloa, Silvestre Frédéric such as Alzheimer’s, Parkinson’s or alanine.alanine. Laboratory of Evolutionary and Amyotrophic lateral sclerosis diseases, Conflict of interest: the authors declare no Adaptive Physiology, Institute of Life, which could be partly related to environ- potential conflict of interest. Earth and Environment, University of mental influences during ELS. Such dis- Namur, Namur, Belgium eases are triggered by a complex interplay Funding: this study was supported by the between genes, ageing, and environmental FRS-FNRS (Fonds de la Recherche conditions plus a random component.4 In Scientifique) PhD fellowship to A. Carion and this context, the need for understanding the a research grant number N°T.0174.14. role played by exposure to NCs has been Abstract growing in importance.5 Of particular inter- Conference presentation: part of this paper was presented at the 2018 EcoBIM meeting, Mangrove rivulus, Kryptolebias mar- est is b-N-methylamino-L-alanine May 22-25, Bordeaux, France. -

FKBP52 Regulates TRPC3-Dependent Ca<Sup>
© 2019. Published by The Company of Biologists Ltd | Journal of Cell Science (2019) 132, jcs231506. doi:10.1242/jcs.231506 RESEARCH ARTICLE FKBP52 regulates TRPC3-dependent Ca2+ signals and the hypertrophic growth of cardiomyocyte cultures Sandra Bandleon1, Patrick P. Strunz1, Simone Pickel2, Oleksandra Tiapko3, Antonella Cellini1, Erick Miranda-Laferte2 and Petra Eder-Negrin1,* ABSTRACT A single TRPC subunit is composed of six transmembrane The transient receptor potential (TRP; C-classical, TRPC) channel domains with a pore-forming loop connecting the transmembrane ‘ ’ TRPC3 allows a cation (Na+/Ca2+) influx that is favored by the domains 5 and 6, a preserved 25 amino acid sequence called a TRP domain and two cytosolic domains, an N-terminal ankyrin repeat stimulation of Gq protein-coupled receptors (GPCRs). An enhanced TRPC3 activity is related to adverse effects, including pathological domain and a C-terminal coiled-coil domain (Eder et al., 2007; Fan hypertrophy in chronic cardiac disease states. In the present study, et al., 2018). The cytosolic domains mediate ion channel formation we identified FK506-binding protein 52 (FKBP52, also known as and are implicated in ion channel regulation and plasma membrane FKBP4) as a novel interaction partner of TRPC3 in the heart. FKBP52 targeting (Eder et al., 2007). Among several protein interaction sites, was recovered from a cardiac cDNA library by a C-terminal TRPC3 the C-terminus of all TRPC subunits harbors a highly conserved fragment (amino acids 742–848) in a yeast two-hybrid screen. proline-rich sequence that corresponds to the binding domain in the Drosophila Downregulation of FKBP52 promoted a TRPC3-dependent photoreceptor channel TRPL for the FK506-binding hypertrophic response in neonatal rat cardiomyocytes (NRCs). -

Table S1. Identified Proteins with Exclusive Expression in Cerebellum of Rats of Control, 10Mg F/L and 50Mg F/L Groups
Table S1. Identified proteins with exclusive expression in cerebellum of rats of control, 10mg F/L and 50mg F/L groups. Accession PLGS Protein Name Group IDa Score Q3TXS7 26S proteasome non-ATPase regulatory subunit 1 435 Control Q9CQX8 28S ribosomal protein S36_ mitochondrial 197 Control P52760 2-iminobutanoate/2-iminopropanoate deaminase 315 Control Q60597 2-oxoglutarate dehydrogenase_ mitochondrial 67 Control P24815 3 beta-hydroxysteroid dehydrogenase/Delta 5-->4-isomerase type 1 84 Control Q99L13 3-hydroxyisobutyrate dehydrogenase_ mitochondrial 114 Control P61922 4-aminobutyrate aminotransferase_ mitochondrial 470 Control P10852 4F2 cell-surface antigen heavy chain 220 Control Q8K010 5-oxoprolinase 197 Control P47955 60S acidic ribosomal protein P1 190 Control P70266 6-phosphofructo-2-kinase/fructose-2_6-bisphosphatase 1 113 Control Q8QZT1 Acetyl-CoA acetyltransferase_ mitochondrial 402 Control Q9R0Y5 Adenylate kinase isoenzyme 1 623 Control Q80TS3 Adhesion G protein-coupled receptor L3 59 Control B7ZCC9 Adhesion G-protein coupled receptor G4 139 Control Q6P5E6 ADP-ribosylation factor-binding protein GGA2 45 Control E9Q394 A-kinase anchor protein 13 60 Control Q80Y20 Alkylated DNA repair protein alkB homolog 8 111 Control P07758 Alpha-1-antitrypsin 1-1 78 Control P22599 Alpha-1-antitrypsin 1-2 78 Control Q00896 Alpha-1-antitrypsin 1-3 78 Control Q00897 Alpha-1-antitrypsin 1-4 78 Control P57780 Alpha-actinin-4 58 Control Q9QYC0 Alpha-adducin 270 Control Q9DB05 Alpha-soluble NSF attachment protein 156 Control Q6PAM1 Alpha-taxilin 161 -
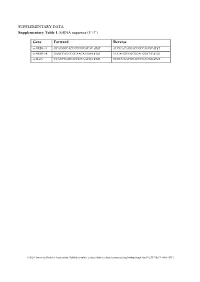
Supplementary Tables and Figures
SUPPLEMENTARY DATA Supplementary Table 1. SiRNA sequence (5’-3’) Gene Forward Reverse si-HRD1-1# GCAUGGCAGUCCUGUACAU dTdT AUGUACAGGACUGCCAUGC dTdT si-HRD1-2# GAGCCAUCCGCAACAUGAA dTdT UUCAUGUUGCGGAUGGCUC dTdT si-MafA CCAUCGAGUACGUCAACGA dTdT UCGUUGACGUACUCGAUGG dTdT ©2020 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-1060/-/DC1 SUPPLEMENTARY DATA Supplementary Table 2. Primer sequences for qRT-PCR (5’-3’) Gene Forward Reverse human HRD1 GCTCACGCCTACTACCTCAAA GCCAGACAAGTCTCTGTGACG mouse mafA AAGCGGCGCACGCTCAAGAA GGTCCCGCTCCTTGGCCAGA mouse insulin1 CACTTCCTACCCCTGCTGG ACCACAAAGATGCTGTTTGACA mouse β-actin AGGCCAACCGTGAAAAGATG AGAGCATAGCCCTCGTAGATGG human β-actin CATGTACGTTGCTATCCAGGC CTCCTTAATGTCACGCACGAT ©2020 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-1060/-/DC1 SUPPLEMENTARY DATA Supplementary Table 3. Primer sequences for ChIP (5’-3’) Gene promoter Forward Reverse mouse Insulin1, 2 GGAACTGTGAAACAGTCCAAGG CCCCCTGGACTTTGCTGTTTG ©2020 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-1060/-/DC1 SUPPLEMENTARY DATA Supplementary Table 4. Primer sequences for PCR (5’-3’) Gene Forward Reverse HRD1-pDsred CCCAAGCTTATGTTCCGCACCGCAGT GGGGTACCCAGTGGGCAACAGGGG HRD1-pCMV- Flag GGGGTACCATGTTCCGCACCGCAGT CCCAAGCTTGTGGGCAACAGGGGACT C HRD1-pCMV-HA GGCCATGGGCCATATGGGATCCTTCC AGGGATGCCACCCGGGGATCCTCAGT GCACCGCAGTGATG GGGCAACAGGGGAC HRD1-N-HA GGCCATGGGCCATATGGGATCCTTCC -
![H00008170-Q01 規格 : [ 10 Ug ] [ 25 Ug ] List All](https://docslib.b-cdn.net/cover/2255/h00008170-q01-10-ug-25-ug-list-all-1052255.webp)
H00008170-Q01 規格 : [ 10 Ug ] [ 25 Ug ] List All
SLC14A2 (Human) Recombinant Protein (Q01) Catalog # : H00008170-Q01 規格 : [ 10 ug ] [ 25 ug ] List All Specification Application Image Product Human SLC14A2 partial ORF ( NP_009094, 40 a.a. - 128 a.a.) Enzyme-linked Immunoabsorbent Assay Description: recombinant protein with GST-tag at N-terminal. Western Blot (Recombinant Sequence: ALPLLEMPEEKDLRSSNEDSHIVKIEKLNERSKRKDDGVAHRDSAGQRCI protein) CLSKAVGYLTGDMKEYRIWLKDKHLALQFIDWVLRGTAQ Antibody Production Host: Wheat Germ (in vitro) Protein Array Theoretical MW 35.53 (kDa): Preparation in vitro wheat germ expression system Method: Purification: Glutathione Sepharose 4 Fast Flow Quality Control 12.5% SDS-PAGE Stained with Coomassie Blue. Testing: Storage Buffer: 50 mM Tris-HCI, 10 mM reduced Glutathione, pH=8.0 in the elution buffer. Storage Store at -80°C. Aliquot to avoid repeated freezing and thawing. Instruction: Note: Best use within three months from the date of receipt of this protein. MSDS: Download Datasheet: Download Applications Enzyme-linked Immunoabsorbent Assay Western Blot (Recombinant protein) Antibody Production Protein Array Page 1 of 2 2016/5/22 Gene Information Entrez GeneID: 8170 GeneBank NM_007163 Accession#: Protein NP_009094 Accession#: Gene Name: SLC14A2 Gene Alias: FLJ16167,HUT2,MGC119566,MGC119567,UT-A2,UT2,UTA,UTR,hUT- A6 Gene solute carrier family 14 (urea transporter), member 2 Description: Omim ID: 601611 Gene Ontology: Hyperlink Gene Summary: In mammalian cells, urea is the chief end-product of nitrogen catabolism and plays an important role in the urinary concentration