Effect of Shifting Cultivation and Charcoal Production on Structure, Dynamic and Above-Ground Biomass in the Angolan Miombo and Dry Woodlands
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Vascular Plant Survey of Vwaza Marsh Wildlife Reserve, Malawi
YIKA-VWAZA TRUST RESEARCH STUDY REPORT N (2017/18) Vascular Plant Survey of Vwaza Marsh Wildlife Reserve, Malawi By Sopani Sichinga ([email protected]) September , 2019 ABSTRACT In 2018 – 19, a survey on vascular plants was conducted in Vwaza Marsh Wildlife Reserve. The reserve is located in the north-western Malawi, covering an area of about 986 km2. Based on this survey, a total of 461 species from 76 families were recorded (i.e. 454 Angiosperms and 7 Pteridophyta). Of the total species recorded, 19 are exotics (of which 4 are reported to be invasive) while 1 species is considered threatened. The most dominant families were Fabaceae (80 species representing 17. 4%), Poaceae (53 species representing 11.5%), Rubiaceae (27 species representing 5.9 %), and Euphorbiaceae (24 species representing 5.2%). The annotated checklist includes scientific names, habit, habitat types and IUCN Red List status and is presented in section 5. i ACKNOLEDGEMENTS First and foremost, let me thank the Nyika–Vwaza Trust (UK) for funding this work. Without their financial support, this work would have not been materialized. The Department of National Parks and Wildlife (DNPW) Malawi through its Regional Office (N) is also thanked for the logistical support and accommodation throughout the entire study. Special thanks are due to my supervisor - Mr. George Zwide Nxumayo for his invaluable guidance. Mr. Thom McShane should also be thanked in a special way for sharing me some information, and sending me some documents about Vwaza which have contributed a lot to the success of this work. I extend my sincere thanks to the Vwaza Research Unit team for their assistance, especially during the field work. -

Agroclimatic Characterization of the Uige Province, Angola Based on the Development of Robusta Coffee
Cultivos Tropicales, 2020, vol. 41, no. 1, e01 enero-marzo ISSN impreso: 0258-5936 Ministerio de Educación Superior. Cuba ISSN digital: 1819-4087 Instituto Nacional de Ciencias Agrícolas http://ediciones.inca.edu.cu Original Article Agroclimatic characterization of the Uige province, Angola based on the development of Robusta Coffee Daniel Fernando Baltazar-da Silva1,2* Mariol Morejón-García1 Andrés Díaz-Pita1 Fernando Manuel de Almeida3 João Ferreira da Costa-Neta4 Vasco Gonçalves4 1Universidad de Pinar del Río, Pinar del Río, Cuba 2Ministerio de Agricultura, Angola 3Universidad de Huamb, Huambo, Angola 4Instituto Nacional do Café. Angola *Author for correspondence. [email protected] ABSTRACT During 2018, this research was carried out with the objective of conducting an agroclimatic characterization based on the development of robust coffee in the Uigé province, Angola. The records of the climatic variables rainfall and temperatures were analyzed, as they are the ones that most influence the development and growth of coffee. The historical-logical method was applied to recover the information about the crop requirements and compare them with the edaphoclimatic conditions of the province. The climatic data of the region were recorded from the observations made in each municipality compatible with the information extracted from the World Meteorological Organization (WMO) site, for the period 1990-2010. Suitability maps for temperatures and rainfall were generated from the use of GIS that allowed the manipulation of thematic information layers. The results allowed us to recognize that the largest area of Uigé province (86.3 %), has climatic conditions for the development of robust coffee, with loss of fitness in the municipalities of the west end of the province, Daniel Fernando Baltazar-da Silva, Mariol Morejón-García, Andrés Díaz-Pita, Fernando Manuel de Almeida, João Ferreira da Costa-Neta y Vasco Gonçalves whose main limitation was rainfall. -

Infected Areas As on 26 January 1989 — Zones Infectées an 26 Janvier 1989 for Criteria Used in Compiling This List, See No
Wkty Epidem Rec No 4 - 27 January 1989 - 26 - Relevé éptdém hebd . N°4 - 27 janvier 1989 (Continued from page 23) (Suite de la page 23) YELLOW FEVER FIÈVRE JAUNE T r in id a d a n d T o b a g o (18 janvier 1989). — Further to the T r i n i t é - e t -T o b a g o (18 janvier 1989). — A la suite du rapport report of yellow fever virus isolation from mosquitos,* 1 the Min concernant l’isolement du virus de la fièvre jaune sur des moustiques,1 le istry of Health advises that there are no human cases and that the Ministère de la Santé fait connaître qu’il n’y a pas de cas humains et que risk to persons in urban areas is epidemiologically minimal at this le risque couru par des personnes habitant en zone urbaine est actuel time. lement minime. Vaccination Vaccination A valid certificate of yellow fever vaccination is N O T required Il n’est PAS exigé de certificat de vaccination anuamarile pour l’en for entry into Trinidad and Tobago except for persons arriving trée à la Trinité-et-Tobago, sauf lorsque le voyageur vient d’une zone from infected areas. (This is a standing position which has infectée. (C’est là une politique permanente qui n ’a pas varié depuis remained unchanged over the last S years.) Sans.) On the other hand, vaccination against yellow fever is recom D’autre part, la vaccination antiamarile est recommandée aux per mended for those persons coming to Trinidad and Tobago who sonnes qui, arrivant à la Trinité-et-Tobago, risquent de se rendre dans may enter forested areas during their stay ; who may be required des zones de -

Investigating the Impact of Fire on the Natural Regeneration of Woody Species in Dry and Wet Miombo Woodland
Investigating the impact of fire on the natural regeneration of woody species in dry and wet Miombo woodland by Paul Mwansa Thesis presented in fulfilment of the requirements for the degree of Master of Science of Forestry and Natural Resource Science in the Faculty of AgriSciences at Stellenbosch University Supervisor: Prof Ben du Toit Co-supervisor: Dr Vera De Cauwer March 2018 Stellenbosch University https://scholar.sun.ac.za Declaration By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. March 2018 Copyright © 2018 Stellenbosch University All rights reserved i Stellenbosch University https://scholar.sun.ac.za Abstract The miombo woodland is an extensive tropical seasonal woodland and dry forest formation in extent of 2.7 million km². The woodland contributes highly to maintenance and improvement of people’s livelihood security and stable growth of national economies. The woodland faces a wide range of disturbances including fire that affect vegetation structure. An investigation into the impact of fire on the natural regeneration of six tree species was conducted along a rainfall gradient. Baikiaea plurijuga, Burkea africana, Guibourtia coleosperma, Pterocarpus angolensis, Schinziophyton rautanenii and Terminalia sericea were selected on basis of being an important timber and/or utilitarian species, and the assumed abundance. The objectives of the study were to examine floristic composition, density and composition of natural regeneration; stand structure and vegetation cover within recently burnt (RB) and recently unburnt (RU) sections of the forest. -

AJAFS E23qcbmp.Pdf
African Journal of Agriculture and Food Science Volume 2, Issue 1, 2019 (pp. 15-27) www.abjournals.org PHYTOCHEMICAL SCREENING, MINERAL DETERMINATION AND ANTIMICROBIAL SCREENING OF THE LEAVES EXTRACTS OF PILIOSTIGMA THONNONGII (MATURED AND YOUNG) LEAVES Ibrahim Isah Laken1, Musah Monday2, Dagaci M.Z1, Mohammed S.H2, Baba H.F2, Umar M.T1 and Usman R.L1 1Department of Chemistry, Ibrahim Badamasi Babangida University Lapai, Niger State, Nigeria 2Deptatment of Chemistry, Niger State College of Education, Minna, Nigeria. ABSTRACT: This paper dwelled on a plant Piliostigma thonningii with reference voucher FNS/0013/ibbu/ 018. The young and matured leaves of Piliostigma thonningii were used in ethno-medicine for the treatment of wounds, ulcers, and gingivitis by some communities in Agaie, Lapai and Bida LGA of Niger State, Nigeria separately. There is no reported scientific register that implicate the equivocal chemical constituent of the leaves. Against this backdrop the young and matured leaves were investigated for chemical constituents, antimicrobial activity and mineral composition. The leaves of the plant were subjected to solvent extraction for ethanolic and aqueous extracts. Both extracts were then subjected to preliminary Phytochemical screening and it was found that the young and matured leaves of the plant both contained alkaloids, flavonoids, Saponins, tannins, Terpenoids, Steroidal nucleus and cardiac glycoside and the absence of Anthraquinone. The matured leave was richer in chemical content than the young leaves. The antimicrobial activity of ethanol and aqueous extracts were studied using isolates of three pathogenic microorganisms and the extracts exhibited activities against two of the three microorganisms with zone of inhibition ranging from 11- 40mm. -

Further Breeding Records for Birds (Aves) in Angola
Durban Natural Science Museum Novitates 36 ANGOLAN BIRD BREEDING RECORDS 1 FURTHER BREEDING RECORDS FOR BIRDS (AVES) IN ANGOLA W. RicHARD J. DeAn1*, URSULA FRAnKe2, GRAnT JOSePH1, FRANCIScO M. GOnÇALVeS3, MicHAeL S.L. MiLLS4,1, SUZAnne J. MiLTOn1, ARA MOnADJeM5 & H. DieTeR OScHADLeUS6 1DST/NRF Centre of Excellence at the Percy FitzPatrick Institute of African Ornithology, University of Cape Town, Rondebosch 7701, South Africa *Author for correspondence: [email protected] 2Tal 34, 80331 Munich, Germany 3ISCED, Department of Natural Sciences, Rua: Sarmento Rodrigues, P.O. Box 230, Lubango, Angola 4A.P. Leventis Ornithological Research Institute, University of Jos, P.O. Box 13404, Jos, Plateau State, Nigeria 5Department of Biological Sciences, University of Swaziland, Private Bag 4, Kwaluseni, Swaziland 6Animal Demography Unit, Department of Zoology, University of Cape Town, Rondebosch 7701, South Africa ean, W.R.J., Franke, U., Joseph, G., Gonçalves, F.M., Mills, M.S.L., Milton, S.J., Monadjem, A. D& Oschadleus, H.D. 2013. Further breeding records for birds (Aves) in Angola. Durban Natural Science Museum Novitates 36: 1-10. Some details of records of nests, eggs and nestlings of 167 (possibly 168) species in the bird collection at Lubango, Angola are given. This includes 23 species for which there were no Angolan breeding records at all, and one possibly new breeding species (Slaty Egret). The data also confirm the breeding of another 20 species strongly suspected of breeding in Angola, but that lacked egg or nestling records. KEYWORDS: Angola, birds, museum collections, breeding. INTRODUcTiOn SYSTeMATIC LiST One of the gaps in our knowledge of the natural history of birds in Taxonomy and order follows Gill & Donsker (2014). -
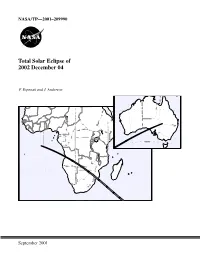
Total Solar Eclipse of 2002 December 4
NASA/TP—2001–209990 Total Solar Eclipse of 2002 December 04 F. Espenak and J. Anderson Central Lat,Lng = -28.0 132.0 P Factor = 0.46 Semi W,H = 0.35 0.28 Offset X,Y = 0.00-0.00 1999 Oct 26 10:40:42 AM High Res World Data [WPD1] WorldMap v2.00, F. Espenak Orthographic Projection Scale = 8.00 mm/° = 1:13915000 Central Lat,Lng = -10.0 26.0 P Factor = 0.31 Semi W,H = 0.70 0.50 Offset X,Y = 0.00-0.00 1999 Oct 26 10:17:57 AM September 2001 The NASA STI Program Office … in Profile Since its founding, NASA has been dedicated to • CONFERENCE PUBLICATION. Collected the advancement of aeronautics and space papers from scientific and technical science. The NASA Scientific and Technical conferences, symposia, seminars, or other Information (STI) Program Office plays a key meetings sponsored or cosponsored by NASA. part in helping NASA maintain this important role. • SPECIAL PUBLICATION. Scientific, techni- cal, or historical information from NASA The NASA STI Program Office is operated by programs, projects, and mission, often con- Langley Research Center, the lead center for cerned with subjects having substantial public NASA’s scientific and technical information. The interest. NASA STI Program Office provides access to the NASA STI Database, the largest collection of • TECHNICAL TRANSLATION. aeronautical and space science STI in the world. English-language translations of foreign scien- The Program Office is also NASA’s institutional tific and technical material pertinent to NASA’s mechanism for disseminating the results of its mission. -

Capítulo 7 Ecossistemas Dominados Por Subarbustos Em Angola
CAPÍTULO 7 ECOSSISTEMAS DOMINADOS POR SUBARBUSTOS EM ANGOLA Paulina Zigelski1, Amândio Gomes1,2 e Manfred Finckh1 Resumo Um mosaico em pequena escala de matas de miombo e de prados abertos sazonalmente inundados é um aspecto típico do fitocório zambe‑ ziano que se estende até às partes oriental e central de Angola. Estes prados albergam as chamadas «árvores subterrâneas» ou subarbustos geoxílicos, uma forma de vida com enormes estruturas lenhosas subterrâneas. Alguns (mas não todos) subarbustos geoxílicos também ocorrem em tipos de mata aberta. Estes icónicos arbustos anões evoluíram em muitas famílias vegetais sob pressões ambientais semelhantes, convertendo assim o fitocório zambe‑ ziano num excelente laboratório para o estudo da evolução. Neste capítulo, reunimos os conhecimentos actuais sobre a distribuição, diversidade, eco‑ logia e história evolutiva dos subarbustos e dos prados geoxílicos de Angola e realçamos o seu valor e desafios de conservação. PalavRas ‑chave Endemismo · Fitocório · Florestas subterrâneas · Geoxilas · Miombo · Vegetação Introdução A vegetação gramínea em zonas abertas é um aspecto comum das paisagens angolanas e é uma parte característica do fitocório zambeziano. As gra‑ míneas são o elemento mais visível destas paisagens no final da estação chuvosa, ao passo que no início desta última muitas espécies lenhosas dos chamados subarbustos geoxílicos ou «árvores subterrâneas» (Davy, 1922; White, 1976) dominam a configuração visual da vegetação. Assim, em vas‑ tas áreas do Centro e do Leste de Angola, os «prados» abertos são de facto 1 Institut für Pflanzwissenschaften und Mikrobiologie, Hamburg Universität, Ohnhorststr. 18, 22609 Hamburg, Deutschland. ² Faculdade de Ciências, Universidade Agostinho Neto, Av. 4 de Fevereiro 71, C. P. 815, Luanda, Angola 158 Biodiversidade de Angola co‑dominados por gramíneas e subarbustos geoxílicos. -

Working Paper Reference
Working Paper Civil wars and state formation: violence and the politics of legitimacy in angola, côte d'ivoire and south sudan PECLARD, Didier, et al. Abstract Civil wars do not only destroy existing political orders. They contribute to shaping new ones, and thereby play a crucial role in dynamics of state formation. This working paper is based on a 2-year research project funded by the Swiss Network of International Studies and conducted by a consortium of five research institutions in Switzerland and Africa. It reflects on the social construction of order and legitimacy during and after violent conflict by focusing on political orders put in place by armed groups, their strategies to legitimize their (violent) action as well as their claim to power, and on the extent to which they strive and manage to institutionalize their military power and transform it into political domination. Drawing on case studies in Angola, Côte d'Ivoire and South Sudan, it shows how strategies of legitimization are central to understanding the politics of armed groups and their relation to the state, how international aid agencies impact on the legitimacy of armed groups and state actors, and how continuities between war and peace, especially in key sectors such as security forces, need to be taken [...] Reference PECLARD, Didier, et al. Civil wars and state formation: violence and the politics of legitimacy in angola, côte d'ivoire and south sudan. Geneva : University of Geneva / Swiss Network of International Studies (SNIS), 2019, 29 p. Available at: http://archive-ouverte.unige.ch/unige:134632 Disclaimer: layout of this document may differ from the published version. -

Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1
Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1 Authors: Jiang, Wei, He, Hua-Jie, Lu, Lu, Burgess, Kevin S., Wang, Hong, et. al. Source: Annals of the Missouri Botanical Garden, 104(2) : 171-229 Published By: Missouri Botanical Garden Press URL: https://doi.org/10.3417/2019337 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/journals/Annals-of-the-Missouri-Botanical-Garden on 01 Apr 2020 Terms of Use: https://bioone.org/terms-of-use Access provided by Kunming Institute of Botany, CAS Volume 104 Annals Number 2 of the R 2019 Missouri Botanical Garden EVOLUTION OF ANGIOSPERM Wei Jiang,2,3,7 Hua-Jie He,4,7 Lu Lu,2,5 POLLEN. 7. NITROGEN-FIXING Kevin S. Burgess,6 Hong Wang,2* and 2,4 CLADE1 De-Zhu Li * ABSTRACT Nitrogen-fixing symbiosis in root nodules is known in only 10 families, which are distributed among a clade of four orders and delimited as the nitrogen-fixing clade. -

2854 ISS Monograph 130.Indd
FFROMROM SSOLDIERSOLDIERS TTOO CCITIZENSITIZENS THE SOCIAL, ECONOMIC AND POLITICAL REINTEGRATION OF UNITA EX-COMBATANTS J GOMES PORTO, IMOGEN PARSONS AND CHRIS ALDEN ISS MONOGRAPH SERIES • No 130, MARCH 2007 CONTENTS ACKNOWLEDGEMENTS iii ABOUT THE AUTHORS v LIST OF ACRONYMS vi INTRODUCTION viii CHAPTER ONE 1 Angola’s Central Highlands: Provincial Characterisation and Fieldwork Review CHAPTER TWO 39 Unita’s Demobilised Soldiers: Portrait of the post-Luena target group CHAPTER THREE 53 The Economic, Social and Political Dimensions of Reintegration: Findings CHAPTER FOUR 79 Surveying for Trends: Correlation of Findings CHAPTER FIVE 109 From Soldiers to Citizens: Concluding Thoughts ENDNOTES 127 BIBLIOGRAPHY 139 ANNEX 145 Survey Questionnaire iii ACKNOWLEDGMENTS The research and publication of this monograph were made possible by the generous funding of the Swedish International Development Cooperation Agency (SIDA), the Swiss Federal Department of Foreign Affairs, and the Norwegian Institute of International Affairs (NUPI), through the African Security Analysis Programme at the ISS. The project “From Soldiers to Citizens: A study of the social, economic and political reintegration of UNITA ex-combatants in post-war Angola” was developed jointly by the African Security Analysis Programme at ISS, the London School of Economics and Political Science (LSE), and the Norwegian Institute for International Affairs (NUPI). In addition, the project established a number of partnerships with Angolan non-governmental organisations (NGOs), including Development -

2.3 Angola Road Network
2.3 Angola Road Network Distance Matrix Travel Time Matrix Road Security Weighbridges and Axle Load Limits For more information on government contact details, please see the following link: 4.1 Government Contact List. Page 1 Page 2 Distance Matrix Uige – River Nzadi bridge 18 m-long and 4 m-wide near the locality of Kitela, north of Songo municipality destroyed during civil war and currently under rehabilitation (news 7/10/2016). Road Details Luanda The Government/MPLA is committed to build 1,100 km of roads in addition to 2,834 km of roads built in 2016 and planned rehabilitation of 7,083 km of roads in addition to 10,219 km rehabilitated in 2016. The Government goals will have also the support from the credit line of the R. of China which will benefit inter-municipality links in Luanda, Uige, Malanje, Cuanza Norte, Cuanza Sul, Benguela, Huambo and Bié provinces. For more information please vitsit the Website of the Ministry of Construction. Zaire Luvo bridge reopened to trucks as of 15/11/2017, this bridge links the municipality of Mbanza Congo with RDC and was closed for 30 days after rehabilitation. Three of the 60 km between MCongo/Luvo require repairs as of 17/11/2017. For more information please visit the Website of Agencia Angola Press. Works of rehabilitation on the road nr, 120 between Mbanza Congo (province Zaire) and the locality of Lukunga (province of Uige) of a distance of 111 km are 60% completed as of 29/9/2017. For more information please visit the Website of Agencia Angola Press.