Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Additional Information
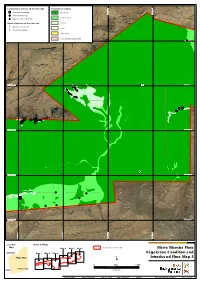
Current Survey Introduced Flora Records Vegetation Condition *Acetosa vesicaria Excellent 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 -

Analysis of Paralogs in Target Enrichment Data Pinpoints Multiple Ancient Polyploidy Events
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.21.261925; this version posted August 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 1 Analysis of paralogs in target enrichment data pinpoints multiple ancient polyploidy events 2 in Alchemilla s.l. (Rosaceae). 3 4 Diego F. Morales-Briones1.2,*, Berit Gehrke3, Chien-Hsun Huang4, Aaron Liston5, Hong Ma6, 5 Hannah E. Marx7, David C. Tank2, Ya Yang1 6 7 1Department of Plant and Microbial Biology, University of Minnesota-Twin Cities, 1445 Gortner 8 Avenue, St. Paul, MN 55108, USA 9 2Department of Biological Sciences and Institute for Bioinformatics and Evolutionary Studies, 10 University of Idaho, 875 Perimeter Drive MS 3051, Moscow, ID 83844, USA 11 3University Gardens, University Museum, University of Bergen, Mildeveien 240, 5259 12 Hjellestad, Norway 13 4State Key Laboratory of Genetic Engineering and Collaborative Innovation Center of Genetics 14 and Development, Ministry of Education Key Laboratory of Biodiversity and Ecological 15 Engineering, Institute of Plant Biology, Center of Evolutionary Biology, School of Life Sciences, 16 Fudan University, Shanghai 200433, China 17 5Department of Botany and Plant Pathology, Oregon State University, 2082 Cordley Hall, 18 Corvallis, OR 97331, USA 19 6Department of Biology, the Huck Institute of the Life Sciences, the Pennsylvania State 20 University, 510D Mueller Laboratory, University Park, PA 16802 USA 21 7Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, MI 22 48109-1048, USA 23 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.21.261925; this version posted August 23, 2020. -

Rail Development Vegetation and Flora Survey
JANUARY 2012 BROCKMAN RESOURCES LIMITED RAIL DEVELOPMENT VEGETATION AND FLORA SURVEY This page has been left blank intentionally BROCKMAN RESOURCES LIMITED RAIL DEVELOPMENT VEGETATION AND FLORA SURVEY Brockman Resources Limited Vegetation and Flora Survey Rail Corridor Document Status Approved for Issue Rev Author Reviewer Date Name Distributed To Date A Rochelle Renee 02/12/2011 Carol Macpherson Glenn Firth 02/12/2011 Haycock Tuckett Carol Macpherson B Carol 21.12.11 Carol Macpherson Glen Firth 23.12.11 Macpherson ecologia Environment (2011). Reproduction of this report in whole or in part by electronic, mechanical or chemical means including photocopying, recording or by any information storage and retrieval system, in any language, is strictly prohibited without the express approval of Brockman Resources Limited and/or ecologia Environment. Restrictions on Use This report has been prepared specifically for Brockman Resources Limited. Neither the report nor its contents may be referred to or quoted in any statement, study, report, application, prospectus, loan, or other agreement document, without the express approval of Brockman Resources Limited and/or ecologia Environment. ecologia Environment 1025 Wellington Street WEST PERTH WA 6005 Phone: 08 9322 1944 Fax: 08 9322 1599 Email: [email protected] December 2011 i Brockman Resources Limited Vegetation and Flora Survey Rail Corridor TABLE OF CONTENTS 1 INTRODUCTION ................................................................................................................ 1 -

Wildlife Travel Burren 2018
The Burren 2018 species list and trip report, 7th-12th June 2018 WILDLIFE TRAVEL The Burren 2018 s 1 The Burren 2018 species list and trip report, 7th-12th June 2018 Day 1: 7th June: Arrive in Lisdoonvarna; supper at Rathbaun Hotel Arriving by a variety of routes and means, we all gathered at Caherleigh House by 6pm, sustained by a round of fresh tea, coffee and delightful home-made scones from our ever-helpful host, Dermot. After introductions and some background to the geology and floral elements in the Burren from Brian (stressing the Mediterranean component of the flora after a day’s Mediterranean heat and sun), we made our way to the Rathbaun, for some substantial and tasty local food and our first taste of Irish music from the three young ladies of Ceolan, and their energetic four-hour performance (not sure any of us had the stamina to stay to the end). Day 2: 8th June: Poulsallach At 9am we were collected by Tony, our driver from Glynn’s Coaches for the week, and following a half-hour drive we arrived at a coastal stretch of species-rich limestone pavement which represented the perfect introduction to the Burren’s flora: a stunningly beautiful mix of coastal, Mediterranean, Atlantic and Arctic-Alpine species gathered together uniquely in a natural rock garden. First impressions were of patchy grassland, sparkling with heath spotted- orchids Dactylorhiza maculata ericetorum and drifts of the ubiquitous and glowing-purple bloody crane’s-bill Geranium sanguineum, between bare rock. A closer look revealed a diverse and colourful tapestry of dozens of flowers - the yellows of goldenrod Solidago virgaurea, kidney-vetch Anthyllis vulneraria, and bird’s-foot trefoil Lotus corniculatus (and its attendant common blue butterflies Polyommatus Icarus), pink splashes of wild thyme Thymus polytrichus and the hairy local subspecies of lousewort Pedicularis sylvatica ssp. -

1 a Survey of the Flora of Remnants Within the Waddy
1 A SURVEY OF THE FLORA OF REMNANTS WITHIN THE WADDY FOREST LAND CONSERVATION DISTRICT Stephen Davies and Phil Ladd for the Waddy Forest Land Conservation District Committee March 2000 2 CONTENTS INTRODUCTION 1 METHODS 3 RESULTS 4 DISCUSSION 56 ACKNOWLEDGEMENTS 59 REFERENCES 60 Appendix 1 - Composite plant list 60 Appendix 2 - Plants found outside the sample sites 67 Appendix 3 - Composite bird list 67 3 INTRODUCTION The Waddy Forest Land Conservation District is about 41,000 hectares and contains 23 substantial land holdings. In 1999 the District received a grant from the National Heritage Trust to survey the flora of its remnant vegetation. Much of this is on private property and the District Committee selected thirty three plots of remnant bushland on private farms to be included in flora survey. The present report is based on visits to these thirty three remnants that lie on 14 of the 23 farms in the district. The surveys are intended to provide information about the biodiversity of the various remnants with the aim of establishing the priority for preservation, by fencing, of the remnants and to determine the value of linking some of them by the planting of corridors of vegetation. At each site the local landholder(s) joined the survey and provided invaluable background information about the history of the remnants. The vegetation of this part of the northern wheatbelt is known to be very diverse. The Marchagee Nature Reserve, lying north west of the District, was surveyed between 1975 and 1977 (Dell et al. 1979). The area was covered by Beard in his vegetation mapping project (Beard 1976), and part of the south of the District was covered in a report on Koobabbie Farm in 1990 (Davies 1990). -

Evolutionary Consequences of Dioecy in Angiosperms: the Effects of Breeding System on Speciation and Extinction Rates
EVOLUTIONARY CONSEQUENCES OF DIOECY IN ANGIOSPERMS: THE EFFECTS OF BREEDING SYSTEM ON SPECIATION AND EXTINCTION RATES by JANA C. HEILBUTH B.Sc, Simon Fraser University, 1996 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES (Department of Zoology) We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA July 2001 © Jana Heilbuth, 2001 Wednesday, April 25, 2001 UBC Special Collections - Thesis Authorisation Form Page: 1 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. The University of British Columbia Vancouver, Canada http://www.library.ubc.ca/spcoll/thesauth.html ABSTRACT Dioecy, the breeding system with male and female function on separate individuals, may affect the ability of a lineage to avoid extinction or speciate. Dioecy is a rare breeding system among the angiosperms (approximately 6% of all flowering plants) while hermaphroditism (having male and female function present within each flower) is predominant. Dioecious angiosperms may be rare because the transitions to dioecy have been recent or because dioecious angiosperms experience decreased diversification rates (speciation minus extinction) compared to plants with other breeding systems. -

Legumes of Wallace Desert Gardens
Bulletin of The Desert Legume Program of The Boyce Thompson Southwestern Arboretum and The University of Arizona Volume 18, Number 2 August 2006 Legumes of Wallace Desert Gardens Pamela Slate standing relationship between our Desert Gardens reviews and Botanic Coordinator organizations, one I see growing ever approves appropriate on-site Wallace Desert Gardens stronger year after year.” projects of mutual benefit. Wallace Desert Gardens is a Matthew B. Johnson non-profit foundation [(502(c)(3) In the mid-1980’s, the Program Manager and Curator under IRS rules] that was created in Wallace’s moved, complete with their Desert Legume Program 1993, well after much of the garden plant collection, from a Paradise was established. Its mission was Valley acre to a Scottsdale The virtues of desert legumes written by HB, as he was fondly subdivision where they purchased captured the attention of H.B. and known, to reflect the original intent of numerous acre-plus lots. At the time, Jocelyn M. Wallace when they first the foundation: HB had “no idea it would be bigger learned of the Desert Legume than a two-acre garden.” Although Program (DELEP) in 1989, about a Wallace Desert Gardens is a he “knew nothing of desert plants year after the program was founded collection of the world’s deserts when he moved to Arizona” in the at the University of Arizona. They plants located at an elevation of early 1980’s, they quickly became his understood the importance of some 2400 feet. Founded by passion. Today the garden legumes’ potential applications H.B. -
![Dissertação [ ] Tese](https://docslib.b-cdn.net/cover/8231/disserta%C3%A7%C3%A3o-tese-978231.webp)
Dissertação [ ] Tese
Universidade Federal de Goiás Instituto de Ciências Biológicas Programa de Pós-Graduação em Biodiversidade Vegetal BERYL EIRENE LUTZ DE MOURA ESTUDOS TAXONÔMICOS E MORFOPOLÍNICOS DAS CUCURBITÁCEAS DO ESTADO DE GOIÁS, BRASIL Goiânia 2017 TERMO DE CIÊNCIA E DE AUTORIZAÇÃO PARA DISPONIBILIZAR AS TESES E DISSERTAÇÕES ELETRÔNICAS NA BIBLIOTECA DIGITAL DA UFG Na qualidade de titular dos direitos de autor, autorizo a Universidade Federal de Goiás (UFG) a disponibilizar, gratuitamente, por meio da Biblioteca Digital de Teses e Dissertações (BDTD/UFG), regulamentada pela Resolução CEPEC nº 832/2007, sem ressarcimento dos direitos autorais, de acordo com a Lei nº 9610/98, o documento conforme permissões assinaladas abaixo, para fins de leitura, impressão e/ou download, a título de divulgação da produção científica brasileira, a partir desta data. 1. Identificação do material bibliográfico: [ X ] Dissertação [ ] Tese 2. Identificação da Tese ou Dissertação Nome completo do autor: BERYL EIRENE LUTZ DE MOURA Título do trabalho: ESTUDOS TAXONÔMICOS E MORFOPOLÍNICOS DAS CUCURBITÁCEAS DO ESTADO DE GOIÁS, BRASIL 3. Informações de acesso ao documento: Concorda com a liberação total do documento [ x ] SIM [ ] NÃO1 Havendo concordância com a disponibilização eletrônica, torna-se imprescindível o envio do(s) arquivo(s) em formato digital PDF da tese ou dissertação. Data: 08/ 05 / 2017 Assinatura do (a) autor (a) 1 Neste caso o documento será embargado por até um ano a partir da data de defesa. A extensão deste prazo suscita justificativa junto à coordenação do curso. Os dados do documento não serão disponibilizados durante o período de embargo. BERYL EIRENE LUTZ DE MOURA ESTUDOS TAXONÔMICOS E MORFOPOLÍNICOS DAS CUCURBITÁCEAS DO ESTADO DE GOIÁS, BRASIL Dissertação apresentada ao Programa de Pós- Graduação em Biodiversidade Vegetal da Universidade Federal de Goiás, como requisito para a obtenção do título de Mestre em Biodiversidade Vegetal Orientadora: Prof.ª Dra. -

Recommendation of Native Species for the Reforestation of Degraded Land Using Live Staking in Antioquia and Caldas’ Departments (Colombia)
UNIVERSITÀ DEGLI STUDI DI PADOVA Department of Land, Environment Agriculture and Forestry Second Cycle Degree (MSc) in Forest Science Recommendation of native species for the reforestation of degraded land using live staking in Antioquia and Caldas’ Departments (Colombia) Supervisor Prof. Lorenzo Marini Co-supervisor Prof. Jaime Polanía Vorenberg Submitted by Alicia Pardo Moy Student N. 1218558 2019/2020 Summary Although Colombia is one of the countries with the greatest biodiversity in the world, it has many degraded areas due to agricultural and mining practices that have been carried out in recent decades. The high Andean forests are especially vulnerable to this type of soil erosion. The corporate purpose of ‘Reforestadora El Guásimo S.A.S.’ is to use wood from its plantations, but it also follows the parameters of the Forest Stewardship Council (FSC). For this reason, it carries out reforestation activities and programs and, very particularly, it is interested in carrying out ecological restoration processes in some critical sites. The study area is located between 2000 and 2750 masl and is considered a low Andean humid forest (bmh-MB). The average annual precipitation rate is 2057 mm and the average temperature is around 11 ºC. The soil has a sandy loam texture with low pH, which limits the amount of nutrients it can absorb. FAO (2014) suggests that around 10 genera are enough for a proper restoration. After a bibliographic revision, the genera chosen were Alchornea, Billia, Ficus, Inga, Meriania, Miconia, Ocotea, Protium, Prunus, Psidium, Symplocos, Tibouchina, and Weinmannia. Two inventories from 2013 and 2019, helped to determine different biodiversity indexes to check the survival of different species and to suggest the adequate characteristics of the individuals for a successful vegetative stakes reforestation. -

Baja California, Mexico, and a Vegetation Map of Colonet Mesa Alan B
Aliso: A Journal of Systematic and Evolutionary Botany Volume 29 | Issue 1 Article 4 2011 Plants of the Colonet Region, Baja California, Mexico, and a Vegetation Map of Colonet Mesa Alan B. Harper Terra Peninsular, Coronado, California Sula Vanderplank Rancho Santa Ana Botanic Garden, Claremont, California Mark Dodero Recon Environmental Inc., San Diego, California Sergio Mata Terra Peninsular, Coronado, California Jorge Ochoa Long Beach City College, Long Beach, California Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Biodiversity Commons, Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Harper, Alan B.; Vanderplank, Sula; Dodero, Mark; Mata, Sergio; and Ochoa, Jorge (2011) "Plants of the Colonet Region, Baja California, Mexico, and a Vegetation Map of Colonet Mesa," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 29: Iss. 1, Article 4. Available at: http://scholarship.claremont.edu/aliso/vol29/iss1/4 Aliso, 29(1), pp. 25–42 ’ 2011, Rancho Santa Ana Botanic Garden PLANTS OF THE COLONET REGION, BAJA CALIFORNIA, MEXICO, AND A VEGETATION MAPOF COLONET MESA ALAN B. HARPER,1 SULA VANDERPLANK,2 MARK DODERO,3 SERGIO MATA,1 AND JORGE OCHOA4 1Terra Peninsular, A.C., PMB 189003, Suite 88, Coronado, California 92178, USA ([email protected]); 2Rancho Santa Ana Botanic Garden, 1500 North College Avenue, Claremont, California 91711, USA; 3Recon Environmental Inc., 1927 Fifth Avenue, San Diego, California 92101, USA; 4Long Beach City College, 1305 East Pacific Coast Highway, Long Beach, California 90806, USA ABSTRACT The Colonet region is located at the southern end of the California Floristic Province, in an area known to have the highest plant diversity in Baja California. -

Geraldton Mt Magnet Road SLK 222.45 Biological Survey May 2012
Main Roads Western Australia Report for Material Source Area: Geraldton Mt Magnet Road SLK 222.45 Biological Survey May 2012 This Report: has been prepared by GHD for Main Roads Western Australia (MRWA) and may only be used and relied on by MRWA for the purpose agreed between GHD and MRWA as set out in section 1.3 of this Report. GHD otherwise disclaims responsibility to any person other than MRWA arising in connection with this Report. GHD also excludes implied warranties and conditions, to the extent legally permissible. The services undertaken by GHD in connection with preparing this Report were limited to those specifically detailed in the Report and are subject to the scope limitations set out in the Report. The opinions, conclusions and any recommendations in this Report are based on conditions encountered and information reviewed at the date of preparation of the Report. GHD has no responsibility or obligation to update this Report to account for events or changes occurring subsequent to the date that the Report was prepared. The opinions, conclusions and any recommendations in this Report are based on assumptions made by GHD described in this Report. GHD disclaims liability arising from any of the assumptions being incorrect. GHD has prepared this Report on the basis of information provided by MRWA and others who provided information to GHD (including Government authorities), which GHD has not independently verified or checked beyond the agreed scope of work. GHD does not accept liability in connection with such unverified information, including errors and omissions in the Report which were caused by errors or omissions in that information.” The opinions, conclusions and any recommendations in this Report are based on information obtained from, and testing undertaken at or in connection with, specific sample points. -

Baseline Biodiversity Report
FINAL Baseline Biodiversity Survey for Potrero Mason Property Prepared for: County of San Diego Department of Parks and Recreation 5500 Overland Avenue Drive, Suite 410 San Diego, California 92123 Contact: Jennifer Price Prepared by: 605 Third Street Encinitas, California 92024 Contact: Brock Ortega DECEMBER 2012 Printed on 30% post-consumer recycled material. Final Baseline Biodiversity Survey Potrero Mason Property TABLE OF CONTENTS Section Page No. LIST OF ACRONYMS ................................................................................................................ V EXECUTIVE SUMMARY .......................................................................................................VII 1.0 INTRODUCTION..............................................................................................................1 1.1 Purpose of the Report.............................................................................................. 1 1.2 MSCP Context ........................................................................................................ 1 2.0 PROPERTY DESCRIPTION ...........................................................................................9 2.1 Project Location ...................................................................................................... 9 2.2 Geographical Setting ............................................................................................... 9 2.3 Geology and Soils ..................................................................................................