Geologic and Geochemical Studies of the New Albany Shale Group (Devonian-Mississippian) in Illinois
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modern Shale Gas Development in the United States: a Primer
U.S. Department of Energy • Office of Fossil Energy National Energy Technology Laboratory April 2009 DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe upon privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. Modern Shale Gas Development in the United States: A Primer Work Performed Under DE-FG26-04NT15455 Prepared for U.S. Department of Energy Office of Fossil Energy and National Energy Technology Laboratory Prepared by Ground Water Protection Council Oklahoma City, OK 73142 405-516-4972 www.gwpc.org and ALL Consulting Tulsa, OK 74119 918-382-7581 www.all-llc.com April 2009 MODERN SHALE GAS DEVELOPMENT IN THE UNITED STATES: A PRIMER ACKNOWLEDGMENTS This material is based upon work supported by the U.S. Department of Energy, Office of Fossil Energy, National Energy Technology Laboratory (NETL) under Award Number DE‐FG26‐ 04NT15455. Mr. Robert Vagnetti and Ms. Sandra McSurdy, NETL Project Managers, provided oversight and technical guidance. -

Long-Range Petroleum Migration in the Illinois Basin CRAIG M
ILLINOIS STATE GEOLOGICAL SURVEY Interior Cratonic Basins, 1991, edited by Reprint Series 1991 - T26 M. W. Leighton, D. R. Kalata, D. F. Oltz, and J. J. Eidel: American Association of Petroleum Geologists Memoir 51,819 p. CHAPTER 26 Long-Range Petroleum Migration in the Illinois Basin CRAIG M. BETHKE DONALD F. OLTZ University of Illinois Illinois State Geological Survey Urbana, Illinois Champaign, Illinois JACKIE D. REED ARCO Oil and Gas Company Plano, Texas Editor's note: No treatment of oil and gas in the Illinois In this section, we define the migration that has basin would be complete without an overview of occurred in the Illinois basin on the basis of shale hydrocarbon migration. The following is a condensed petrographic observations and geochemical correla version, made especially for this volume, of an article tions. We emphasize the origin of oils found in shallow that will appear in the American Association of reservoirs, mostly in Silurian strata to the north and Petroleum Geologists Bulletin in 1991. northwest of the central basin (Figure 26-2), because these oils seem to have migrated farthest from source beds. We show that these oils, which are produced from reservoirs more than 125 mi (200 km) from the basin's depocenter, were derived from Devonian source rocks in the deep basin. We then use quantitative models of the basin's paleohydrology to INTRODUCTION investigate the past subsurface conditions that drove the migration. We conclude that long-range migration The distribution of petroleum production from the was not related to processes occurring as the basin Illinois basin extends considerably beyond the area subsided and infilled. -

Geology of the Devonian Marcellus Shale—Valley and Ridge Province
Geology of the Devonian Marcellus Shale—Valley and Ridge Province, Virginia and West Virginia— A Field Trip Guidebook for the American Association of Petroleum Geologists Eastern Section Meeting, September 28–29, 2011 Open-File Report 2012–1194 U.S. Department of the Interior U.S. Geological Survey Geology of the Devonian Marcellus Shale—Valley and Ridge Province, Virginia and West Virginia— A Field Trip Guidebook for the American Association of Petroleum Geologists Eastern Section Meeting, September 28–29, 2011 By Catherine B. Enomoto1, James L. Coleman, Jr.1, John T. Haynes2, Steven J. Whitmeyer2, Ronald R. McDowell3, J. Eric Lewis3, Tyler P. Spear3, and Christopher S. Swezey1 1U.S. Geological Survey, Reston, VA 20192 2 James Madison University, Harrisonburg, VA 22807 3 West Virginia Geological and Economic Survey, Morgantown, WV 26508 Open-File Report 2012–1194 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior Ken Salazar, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia: 2012 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted material contained within this report. -

U.S. Shale Gas
U.S. Shale Gas An Unconventional Resource. Unconventional Challenges. WHITE PAPER U.S. Shale Gas An Unconventional Resource . Unconventional Challenges . Executive Summary Current increasing demand and lagging supply mean high prices for both oil and gas, making exploitation of North American unconventional gas plays suddenly far more lucrative for producers. One of the most important such plays to emerge has been U.S. shale gas, with current recoverable reserves conservatively estimated at 500 to 1,000 trillion cubic feet. Hydraulic fracturing and horizontal drilling are the key enabling technologies that first made recovery of shale gas economically viable with their introduction in the Barnett Shale of Texas during the 1990s. However, a comparison of the currently hottest shale plays makes it clear that, after two decades of development and several iterations of the learning curve, best practices are application-dependent and must evolve locally. That said, a review of current trends in these hot plays indicates that, in many cases, the impact of high-drilling density required to develop continuous gas accumulations can be minimized through early and better identification of the accumulation type and size, well- designed access and transportation networks, and cooperative planning and construction efforts, when possible. U.S. Shale Gas Geographic Potential Across the U.S., from the West Coast to the Northeast, some 19 geographic basins are recognized sources of shale gas, where an estimated 35,000 wells were drilled in 2006. Presently, significant commercial gas shale production occurs in the Barnett Shale in the Fort Worth Basin, Lewis Shale in the San Juan Basin, Antrim Shale in the Michigan Basin, Marcellus Shale and others in the Appalachian Basin, and New Albany Shale in the Illinois Basin. -

Mineral Resources of the Illinois Basin in the Context of Basin Evolution
Mineral Resources of the Illinois Basin in the Context of Basin Evolution St. Louis, Missouri, January 22-23,1992 Program and Abstracts Edited by Martin B. Goldhaber and J. James Eidel U.S. Geological Survey Open-File Report 92-1 This report is preliminary and has not been edited or reviewed for conformity with U.S. Geological Survey, Illinois State Geological Survey, Kentucky Geological Survey, Missouri Division of Geology and Land Survey, and Indiana Geological Survey standards. PREFACE The mineral resources of the U.S. midcontinent were instrumental to the development of the U.S. economy. Mineral resources are an important and essential component of the current economy and will continue to play a vital role in the future. Mineral resources provide essential raw materials for the goods consumed by industry and the public. To assess the availability of mineral resources and contribute to the abib'ty to locate and define mineral resources, the U.S. Geological Survey (USGS) has undertaken two programs in cooperation with the State Geological Surveys in the midcontinent region. In 1975, under the Conterminous U.S. Mineral Assessment Program (CUSMAP) work began on the Rolla 1° X 2° Quadrangle at a scale of 1:250,000 and was continued in the adjacent Springfield, Harrison, Joplin, and Paducah quadrangles across southern Missouri, Kansas, Illinois, Arkansas, and Oklahoma. Public meetings were held in 1981 to present results from the Rolla CUSMAP and in 1985 for the Springfield CUSMAP. In 1984, the Midcontinent Strategic and Critical Minerals Project (SCMP) was initiated by the USGS and the State Geological Surveys of 16 states to map and compile data at 1:1,000,000 scale and conduct related topical studies for the area from latitude 36° to 46° N. -

Permian Basin, West Texas and Southeastern New Mexico
Report of Investigations No. 201 Stratigraphic Analysis of the Upper Devonian Woodford Formation, Permian Basin, West Texas and Southeastern New Mexico John B. Comer* *Current address Indiana Geological Survey Bloomington, Indiana 47405 1991 Bureau of Economic Geology • W. L. Fisher, Director The University of Texas at Austin • Austin, Texas 78713-7508 Contents Abstract ..............................................................................................................................1 Introduction ..................................................................................................................... 1 Methods .............................................................................................................................3 Stratigraphy .....................................................................................................................5 Nomenclature ...................................................................................................................5 Age and Correlation ........................................................................................................6 Previous Work .................................................................................................................6 Western Outcrop Belt ......................................................................................................6 Central Texas ...................................................................................................................7 Northeastern Oklahoma -

Reservoir Quality and Diagenetic
U.S. Department of the Interior U.S. Geological Survey Reservoir Quality and Diagenetic Evolution of Upper Mississippian Rocks in the Illinois Basin: Influence of a Regional Hydrothermal Fluid-Flow Event During Late Diagenesis U.S. Geological Survey Professional Paper 1597 Availability of Publications of the U.S. Geological Survey Order U.S. Geological Survey (USGS) publications from the Documents. Check or money order must be payable to the offices listed below. Detailed ordering instructions, along with Superintendent of Documents. Order by mail from— prices of the last offerings, are given in the current-year issues of the catalog "New Publications of the U.S. Geological Superintendent of Documents Survey." Government Printing Office Washington, DC 20402 Books, Maps, and Other Publications Information Periodicals By Mail Many Information Periodicals products are available through Books, maps, and other publications are available by mail the systems or formats listed below: from— Printed Products USGS Information Services Box 25286, Federal Center Printed copies of the Minerals Yearbook and the Mineral Com Denver, CO 80225 modity Summaries can be ordered from the Superintendent of Publications include Professional Papers, Bulletins, Water- Documents, Government Printing Office (address above). Supply Papers, Techniques of Water-Resources Investigations, Printed copies of Metal Industry Indicators and Mineral Indus Circulars, Fact Sheets, publications of general interest, single try Surveys can be ordered from the Center for Disease Control copies of permanent USGS catalogs, and topographic and and Prevention, National Institute for Occupational Safety and thematic maps. Health, Pittsburgh Research Center, P.O. Box 18070, Pitts burgh, PA 15236-0070. Over the Counter Mines FaxBack: Return fax service Books, maps, and other publications of the U.S. -
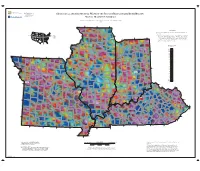
Geological and Geophysical Maps of the Illinois Basin-Ozark Dome Region
Prairie Research Institute Illinois State Geological Survey 615 East Peabody Drive GEOLOGICAL AND GEOPHYSICAL MAPS OF THE ILLINOIS BASIN–OZARK DOME REGION Champaign, Illinois 61820-6918 (217) 244-2414 http://www.isgs.illinois.edu MAP 10: MAGNETIC ANOMALY Digitally compiled by Curtis C. Abert, Stephen Marshak, and Timothy H. Larson 2016 90° 89° 88° Data Source 90° 0' 0" W Raster cell size is approximately 1,026 meters. Data were extracted from the fol- lowing source: 40° 0' 0" N STATES Bankey, V., A. Cuevas, D. Daniels, C.A. Finn, I. Hernandez, P. Hill, R. Kucks, W. 1 Missouri 42° 42° Miles, M. Pilkington, C. Roberts, W. Roest, V. Rystrom, S. Shearer, S. Snyder, 2 3 2 Illinois 3 Indiana 85° R. Sweeney, J. Velez, J.D. Phillips, and D. Ravat, 2002, Digital data grids for 1 4 86° 4 Kentucky Chicago! the magnetic anomaly map of North America: Denver, Colorado, U.S. Geo- 87° logical Survey, Open-File Report 02-414, http://mrdata.usgs.gov/geophysics/ aeromag.html (accessed September 9, 2015). 91° Magnetic Anomaly Nanotesla (nT) 400 200 41° 150 41° 100 90 95° 94° 70 93° 92° 50 30 20 10 0 40° Ϫ10 40° Ϫ20 Ϫ30 Ϫ40 ! Indianapolis Ϫ60 Ϫ80 Ϫ100 Ϫ125 95° Ϫ175 Ϫ200 Ϫ300 ! Kansas City 39° 39° 84° 83° St. Louis! Louisville ! 38° 38° 82° 82° 37° 37° 83° 85° 84° 87° 86° 94° 93° 92° 91° 89° 88° 36° 36° 90° 1: 1,500,000 Vector base data from 2009 Esri Data and Maps Digital cartography by Curtis C. Abert and Jennifer E. -

Mississippian Oolites and Petroleum Reservoirs in the United States—An Overview
Chapter 1 • Mississippian Oolites and Petroleum Reservoirs in the United States—An Overview Brian D. Keith Indiana Geological Survey and Indiana University Bloomington, Indiana, USA Charles W. Zuppann Indiana Geological Survey Bloomington, Indiana, USA • ABSTRACT A coincidence of tectonic, eustatic, and geochemical conditions resulted in substantial deposits of oolitic limestone during later Mississippian time in the continental United States. These oolitic limestones have formed petrole um reservoirs with favorable primary and secondary recovery characteris tics. Significant potential reserves in stratigraphic traps remain to be discovered and developed in these reservoirs. INTRODUCTION oolitic limestone deposition on the North American continent was during the Mississippian. Mississippian rocks of the continental U.S. have With the recent emphasis on global-scale geologic been the subject of two previous compilation vol processes, we recognize that attention must be given umes by the USGS (Craig and Connor, 1979; U.S. to Mississippian oolitic deposition on a broader scale, Geological Survey, 1979), both concentrating on the seeking to understand the factors that influenced and Mississippian system as a whole, the former on controlled oolitic deposition at that time. A prerequi Mississippian and Pennsylvanian stratigraphy and site to interpreting worldwide depositional patterns the latter on the paleotectonic history of the of Mississippian oolitic rocks is to study these Mississippian. However, there has not been a publi deposits at both the regional and the continental cation specifically emphasizing Mississippian oolitic scales. The abundant occurrence and widespread dis rocks. Geologists have reported oolitic limestones in tribution of Mississippian oolitic rocks, and the Mississippian rocks in many areas of the United wealth of available subsurface information, make the States for years, but Wilson (1975, p. -

Guide to the Geology of the Galena Area
5 (^$UiAA>*>M C 3 Guide to the Geology of the Galena Area Jo Daviess County, Illinois Lafayette County, Wisconsin David L. Reinertsen Field Trip Guidebook 1992B May 16, 1992 Department of Energy and Natural Resources ILLINOIS STATE GEOLOGICAL SURVEY LIBRARY. Cover photos by D. L. Reinertsen Clockwise from upper left: Silurian dolomite cap on Scales Mound, early crevice mine south of Galena near the Mississippi River, and downtown Galena as viewed from the old Galena High School. Geological Science Field Trips The Educational Extension Unit of the Illinois State Geological Survey (ISGS) conducts four free tours each year to acquaint the public with the rocks, mineral resources, and landscapes of various regions of the state and the geological processes that have led to their origin. Each trip is an all-day excursion through one or more Illinois counties. Frequent stops are made to explore interesting phenomena, explain the processes that shape our environment, discuss principles of earth science, and collect rocks and fossils. People of all ages and interests are welcome. The trips are especially helpful to teachers who prepare earth science units. Grade school students are welcome, but each must be accompanied by a parent or guardian. High school science classes should be supervised by at least one adult for each ten students. A list of guidebooks of earlier field trips for planning class tours and private outings may be obtained by contacting the Educational Extension Unit, Illinois State Geological Survey, Natural Resources Building, 615 East Peabody Drive, Champaign, IL 61820. Telephone: (217) 244- 2407 or 333-7372. -

Overview of Coal Mine Ground Control Issues in the Illinois Basin
Overview of coal mine ground control issues in the Illinois Basin G.M. Molinda, C. Mark, D.M. Pappas and T.M. Klemetti Gerologist, mining engineer, civil engineer and mining engineer, respectively, National Institute for Occupational Safety and Health (NIOSH), Pittsburgh, Pennsylvania Abstract Some of the most difficult coal mine roof in the United States can be found in the Illinois Basin. Factors contributing to the high roof fall rate include weak, moisture-sensitive roof rock; high horizontal stress; and limited longwall mining. The depth of cover ranges from 27 to 300 m (90 to 1,000 ft), and roof dam- age from horizontal stress can be severe. Moisture-sensitive roof rock, which contributes to roof skin deterioration and roof fall, is common above the Springfield-Harrisburg Herrin #5 and #6 seams in the Illinois Basin. The roof fall rate increases significantly in the humid summer months. Using laboratory and field studies, the National Institute for Occupational Safety and Health (NIOSH) has shown that highly moisture-sensitive roof rock can be directly correlated to poor roof conditions. Controlling the skin is the key to reducing rock fall injuries, and roof screening is, by far, the best remedy. Illinois Basin coal operators have been successful in reducing the number of rock fall injuries in recent years. NIOSH has documented best practices for screen installation, which has resulted in safe, efficient operations. Other solutions to skin failure include the use of denser five bolts/row patterns to reduce spans between bolts; systematic supplemental support in intersections; straps and large pans to protect operators; and air conditioning to remove moisture from the intake air. -

B2150-B FRONT Final
Bedrock Geology of the Paducah 1°×2° CUSMAP Quadrangle, Illinois, Indiana, Kentucky, and Missouri By W. John Nelson THE PADUCAH CUSMAP QUADRANGLE: RESOURCE AND TOPICAL INVESTIGATIONS Martin B. Goldhaber, Project Coordinator T OF EN TH TM E U.S. GEOLOGICAL SURVEY BULLETIN 2150–B R I A N P T E E R D . I O S . R A joint study conducted in collaboration with the Illinois State Geological U Survey, the Indiana Geological Survey, the Kentucky Geological Survey, and the Missouri M 9 Division of Geology and Land Survey A 8 4 R C H 3, 1 UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1998 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Mark Schaefer, Acting Director For sale by U.S. Geological Survey, Information Services Box 25286, Federal Center Denver, CO 80225 Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government Library of Congress Cataloging-in-Publication Data Nelson, W. John Bedrock geology of the Paducah 1°×2° CUSMAP Quadrangle, Illinois, Indiana, Ken- tucky, and Missouri / by W. John Nelson. p. cm.—(U.S. Geological Survey bulletin ; 2150–B) (The Paducah CUSMAP Quadrangle, resource and topical investigations ; B) Includes bibliographical references. Supt. of Docs. no. : I 19.3:2150–B 1. Geology—Middle West. I. Title. II. Series. III. Series: The Paducah CUSMAP Quadrangle, resource and topical investigations ; B QE75.B9 no. 2150–B [QE78.7] [557.3 s—dc21 97–7724 [557.7] CIP CONTENTS Abstract ..........................................................................................................................