Reservoir Quality and Diagenetic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Long-Range Petroleum Migration in the Illinois Basin CRAIG M
ILLINOIS STATE GEOLOGICAL SURVEY Interior Cratonic Basins, 1991, edited by Reprint Series 1991 - T26 M. W. Leighton, D. R. Kalata, D. F. Oltz, and J. J. Eidel: American Association of Petroleum Geologists Memoir 51,819 p. CHAPTER 26 Long-Range Petroleum Migration in the Illinois Basin CRAIG M. BETHKE DONALD F. OLTZ University of Illinois Illinois State Geological Survey Urbana, Illinois Champaign, Illinois JACKIE D. REED ARCO Oil and Gas Company Plano, Texas Editor's note: No treatment of oil and gas in the Illinois In this section, we define the migration that has basin would be complete without an overview of occurred in the Illinois basin on the basis of shale hydrocarbon migration. The following is a condensed petrographic observations and geochemical correla version, made especially for this volume, of an article tions. We emphasize the origin of oils found in shallow that will appear in the American Association of reservoirs, mostly in Silurian strata to the north and Petroleum Geologists Bulletin in 1991. northwest of the central basin (Figure 26-2), because these oils seem to have migrated farthest from source beds. We show that these oils, which are produced from reservoirs more than 125 mi (200 km) from the basin's depocenter, were derived from Devonian source rocks in the deep basin. We then use quantitative models of the basin's paleohydrology to INTRODUCTION investigate the past subsurface conditions that drove the migration. We conclude that long-range migration The distribution of petroleum production from the was not related to processes occurring as the basin Illinois basin extends considerably beyond the area subsided and infilled. -

Geology of the Devonian Marcellus Shale—Valley and Ridge Province
Geology of the Devonian Marcellus Shale—Valley and Ridge Province, Virginia and West Virginia— A Field Trip Guidebook for the American Association of Petroleum Geologists Eastern Section Meeting, September 28–29, 2011 Open-File Report 2012–1194 U.S. Department of the Interior U.S. Geological Survey Geology of the Devonian Marcellus Shale—Valley and Ridge Province, Virginia and West Virginia— A Field Trip Guidebook for the American Association of Petroleum Geologists Eastern Section Meeting, September 28–29, 2011 By Catherine B. Enomoto1, James L. Coleman, Jr.1, John T. Haynes2, Steven J. Whitmeyer2, Ronald R. McDowell3, J. Eric Lewis3, Tyler P. Spear3, and Christopher S. Swezey1 1U.S. Geological Survey, Reston, VA 20192 2 James Madison University, Harrisonburg, VA 22807 3 West Virginia Geological and Economic Survey, Morgantown, WV 26508 Open-File Report 2012–1194 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior Ken Salazar, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia: 2012 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted material contained within this report. -

Geologic and Geochemical Studies of the New Albany Shale Group (Devonian-Mississippian) in Illinois
557 3M SuMr IL6of 1980-2 Final Report Geologic and Geochemical Studies of the New Albany Shale Group (Devonian-Mississippian) in Illinois R. E. Bergstrom and N. F. ShimP/ Principal Invistigators Robert M. Cluff, Project Coordinator Illinois State Geological Survey Open File Series 1980-2 June 30, 1980 Illinois State Geological Survey Urbana, IL 61801 Prepared for U.S. Department of Energy Morgantown Energy Technology Center Morgantown, WV 2 6505 Contract: U.S. Department of Energy DE-AC21-76ET12142 , Note to readers: Printouts of the data contained on the computer file and copies of the structure map mentioned in this report can be obtained from the Illinois State Geological Survey upon written request, This report was prepared as an account of work partially sponsored by the U.S. Government. Neither the United States nor the United States Depart- ment of Energy, nor any of their employees , contractors, subcontractors or employees makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. '^'NOIS STATE QEOLOQICAL SURVEY 3 305 Final Report Geologic and Geochemical Studies of the New Albany Shale Group (Devonian-Mississippian) in Illinois R. E. Bergstrom and N. F. Shimp, Principal Invistigators Robert M. Cluff, Project Coordinator Illinois State Geological Survey Open File Series 1980-2 SEP 1 1 1996 |L uluu ourWtY June 30, 1980 Illinois State Geological Survey Urbana, IL 61801 Prepared for U.S. Department of Energy Morgantown Energy Technology Center Morgantown, WV 2 6505 Contract: U.S. -

Mineral Resources of the Illinois Basin in the Context of Basin Evolution
Mineral Resources of the Illinois Basin in the Context of Basin Evolution St. Louis, Missouri, January 22-23,1992 Program and Abstracts Edited by Martin B. Goldhaber and J. James Eidel U.S. Geological Survey Open-File Report 92-1 This report is preliminary and has not been edited or reviewed for conformity with U.S. Geological Survey, Illinois State Geological Survey, Kentucky Geological Survey, Missouri Division of Geology and Land Survey, and Indiana Geological Survey standards. PREFACE The mineral resources of the U.S. midcontinent were instrumental to the development of the U.S. economy. Mineral resources are an important and essential component of the current economy and will continue to play a vital role in the future. Mineral resources provide essential raw materials for the goods consumed by industry and the public. To assess the availability of mineral resources and contribute to the abib'ty to locate and define mineral resources, the U.S. Geological Survey (USGS) has undertaken two programs in cooperation with the State Geological Surveys in the midcontinent region. In 1975, under the Conterminous U.S. Mineral Assessment Program (CUSMAP) work began on the Rolla 1° X 2° Quadrangle at a scale of 1:250,000 and was continued in the adjacent Springfield, Harrison, Joplin, and Paducah quadrangles across southern Missouri, Kansas, Illinois, Arkansas, and Oklahoma. Public meetings were held in 1981 to present results from the Rolla CUSMAP and in 1985 for the Springfield CUSMAP. In 1984, the Midcontinent Strategic and Critical Minerals Project (SCMP) was initiated by the USGS and the State Geological Surveys of 16 states to map and compile data at 1:1,000,000 scale and conduct related topical studies for the area from latitude 36° to 46° N. -
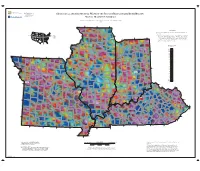
Geological and Geophysical Maps of the Illinois Basin-Ozark Dome Region
Prairie Research Institute Illinois State Geological Survey 615 East Peabody Drive GEOLOGICAL AND GEOPHYSICAL MAPS OF THE ILLINOIS BASIN–OZARK DOME REGION Champaign, Illinois 61820-6918 (217) 244-2414 http://www.isgs.illinois.edu MAP 10: MAGNETIC ANOMALY Digitally compiled by Curtis C. Abert, Stephen Marshak, and Timothy H. Larson 2016 90° 89° 88° Data Source 90° 0' 0" W Raster cell size is approximately 1,026 meters. Data were extracted from the fol- lowing source: 40° 0' 0" N STATES Bankey, V., A. Cuevas, D. Daniels, C.A. Finn, I. Hernandez, P. Hill, R. Kucks, W. 1 Missouri 42° 42° Miles, M. Pilkington, C. Roberts, W. Roest, V. Rystrom, S. Shearer, S. Snyder, 2 3 2 Illinois 3 Indiana 85° R. Sweeney, J. Velez, J.D. Phillips, and D. Ravat, 2002, Digital data grids for 1 4 86° 4 Kentucky Chicago! the magnetic anomaly map of North America: Denver, Colorado, U.S. Geo- 87° logical Survey, Open-File Report 02-414, http://mrdata.usgs.gov/geophysics/ aeromag.html (accessed September 9, 2015). 91° Magnetic Anomaly Nanotesla (nT) 400 200 41° 150 41° 100 90 95° 94° 70 93° 92° 50 30 20 10 0 40° Ϫ10 40° Ϫ20 Ϫ30 Ϫ40 ! Indianapolis Ϫ60 Ϫ80 Ϫ100 Ϫ125 95° Ϫ175 Ϫ200 Ϫ300 ! Kansas City 39° 39° 84° 83° St. Louis! Louisville ! 38° 38° 82° 82° 37° 37° 83° 85° 84° 87° 86° 94° 93° 92° 91° 89° 88° 36° 36° 90° 1: 1,500,000 Vector base data from 2009 Esri Data and Maps Digital cartography by Curtis C. Abert and Jennifer E. -

Mississippian Oolites and Petroleum Reservoirs in the United States—An Overview
Chapter 1 • Mississippian Oolites and Petroleum Reservoirs in the United States—An Overview Brian D. Keith Indiana Geological Survey and Indiana University Bloomington, Indiana, USA Charles W. Zuppann Indiana Geological Survey Bloomington, Indiana, USA • ABSTRACT A coincidence of tectonic, eustatic, and geochemical conditions resulted in substantial deposits of oolitic limestone during later Mississippian time in the continental United States. These oolitic limestones have formed petrole um reservoirs with favorable primary and secondary recovery characteris tics. Significant potential reserves in stratigraphic traps remain to be discovered and developed in these reservoirs. INTRODUCTION oolitic limestone deposition on the North American continent was during the Mississippian. Mississippian rocks of the continental U.S. have With the recent emphasis on global-scale geologic been the subject of two previous compilation vol processes, we recognize that attention must be given umes by the USGS (Craig and Connor, 1979; U.S. to Mississippian oolitic deposition on a broader scale, Geological Survey, 1979), both concentrating on the seeking to understand the factors that influenced and Mississippian system as a whole, the former on controlled oolitic deposition at that time. A prerequi Mississippian and Pennsylvanian stratigraphy and site to interpreting worldwide depositional patterns the latter on the paleotectonic history of the of Mississippian oolitic rocks is to study these Mississippian. However, there has not been a publi deposits at both the regional and the continental cation specifically emphasizing Mississippian oolitic scales. The abundant occurrence and widespread dis rocks. Geologists have reported oolitic limestones in tribution of Mississippian oolitic rocks, and the Mississippian rocks in many areas of the United wealth of available subsurface information, make the States for years, but Wilson (1975, p. -

Guide to the Geology of the Galena Area
5 (^$UiAA>*>M C 3 Guide to the Geology of the Galena Area Jo Daviess County, Illinois Lafayette County, Wisconsin David L. Reinertsen Field Trip Guidebook 1992B May 16, 1992 Department of Energy and Natural Resources ILLINOIS STATE GEOLOGICAL SURVEY LIBRARY. Cover photos by D. L. Reinertsen Clockwise from upper left: Silurian dolomite cap on Scales Mound, early crevice mine south of Galena near the Mississippi River, and downtown Galena as viewed from the old Galena High School. Geological Science Field Trips The Educational Extension Unit of the Illinois State Geological Survey (ISGS) conducts four free tours each year to acquaint the public with the rocks, mineral resources, and landscapes of various regions of the state and the geological processes that have led to their origin. Each trip is an all-day excursion through one or more Illinois counties. Frequent stops are made to explore interesting phenomena, explain the processes that shape our environment, discuss principles of earth science, and collect rocks and fossils. People of all ages and interests are welcome. The trips are especially helpful to teachers who prepare earth science units. Grade school students are welcome, but each must be accompanied by a parent or guardian. High school science classes should be supervised by at least one adult for each ten students. A list of guidebooks of earlier field trips for planning class tours and private outings may be obtained by contacting the Educational Extension Unit, Illinois State Geological Survey, Natural Resources Building, 615 East Peabody Drive, Champaign, IL 61820. Telephone: (217) 244- 2407 or 333-7372. -

Overview of Coal Mine Ground Control Issues in the Illinois Basin
Overview of coal mine ground control issues in the Illinois Basin G.M. Molinda, C. Mark, D.M. Pappas and T.M. Klemetti Gerologist, mining engineer, civil engineer and mining engineer, respectively, National Institute for Occupational Safety and Health (NIOSH), Pittsburgh, Pennsylvania Abstract Some of the most difficult coal mine roof in the United States can be found in the Illinois Basin. Factors contributing to the high roof fall rate include weak, moisture-sensitive roof rock; high horizontal stress; and limited longwall mining. The depth of cover ranges from 27 to 300 m (90 to 1,000 ft), and roof dam- age from horizontal stress can be severe. Moisture-sensitive roof rock, which contributes to roof skin deterioration and roof fall, is common above the Springfield-Harrisburg Herrin #5 and #6 seams in the Illinois Basin. The roof fall rate increases significantly in the humid summer months. Using laboratory and field studies, the National Institute for Occupational Safety and Health (NIOSH) has shown that highly moisture-sensitive roof rock can be directly correlated to poor roof conditions. Controlling the skin is the key to reducing rock fall injuries, and roof screening is, by far, the best remedy. Illinois Basin coal operators have been successful in reducing the number of rock fall injuries in recent years. NIOSH has documented best practices for screen installation, which has resulted in safe, efficient operations. Other solutions to skin failure include the use of denser five bolts/row patterns to reduce spans between bolts; systematic supplemental support in intersections; straps and large pans to protect operators; and air conditioning to remove moisture from the intake air. -

B2150-B FRONT Final
Bedrock Geology of the Paducah 1°×2° CUSMAP Quadrangle, Illinois, Indiana, Kentucky, and Missouri By W. John Nelson THE PADUCAH CUSMAP QUADRANGLE: RESOURCE AND TOPICAL INVESTIGATIONS Martin B. Goldhaber, Project Coordinator T OF EN TH TM E U.S. GEOLOGICAL SURVEY BULLETIN 2150–B R I A N P T E E R D . I O S . R A joint study conducted in collaboration with the Illinois State Geological U Survey, the Indiana Geological Survey, the Kentucky Geological Survey, and the Missouri M 9 Division of Geology and Land Survey A 8 4 R C H 3, 1 UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1998 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Mark Schaefer, Acting Director For sale by U.S. Geological Survey, Information Services Box 25286, Federal Center Denver, CO 80225 Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government Library of Congress Cataloging-in-Publication Data Nelson, W. John Bedrock geology of the Paducah 1°×2° CUSMAP Quadrangle, Illinois, Indiana, Ken- tucky, and Missouri / by W. John Nelson. p. cm.—(U.S. Geological Survey bulletin ; 2150–B) (The Paducah CUSMAP Quadrangle, resource and topical investigations ; B) Includes bibliographical references. Supt. of Docs. no. : I 19.3:2150–B 1. Geology—Middle West. I. Title. II. Series. III. Series: The Paducah CUSMAP Quadrangle, resource and topical investigations ; B QE75.B9 no. 2150–B [QE78.7] [557.3 s—dc21 97–7724 [557.7] CIP CONTENTS Abstract .......................................................................................................................... -

Grand Tower Limestone (Devonian) of Southern Illinois
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Illinois Digital Environment for Access to Learning and Scholarship... STATE OF ILLINOIS DEPARTMENT OF REGISTRATION AND EDUCATION GRAND TOWER LIMESTONE (DEVONIAN) OF SOUTHERN ILLINOIS Wayne F. Meents David H. Swann ILLINOIS STATE GEOLOGICAL SURVEY John C. Frye, Chief URBANA CIRCULAR 389 1965 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/grandtowerlimest389meen GRAND TOWER LIMESTONE (DEVONIAN) OF SOUTHERN ILLINOIS Wayne F. Meents and David H. Swann ABSTRACT The Grand Tower Limestone has been the source of over a hundred million barrels of oil in Illinois, the bulk of the Devo- nian production of the state. The formation underlies most of southern and central Illinois and extends into Missouri, Iowa, Indiana, and Kentucky, where its equivalents are the Cooper, Wapsipinicon, and Jeffersonville Limestones. The Grand Tower has a maximum thickness of a little more than 200 feet. It is a sandy, but otherwise pure, carbonate unit that forms approxi- mately the lower half of the Middle Devonian carbonate sequence of the region. TheGrand Tower is dominantly fossiliferous lime- stone in southern Illinois, dolomite in most of central Illinois, and lithographic limestone where it pinches out in north-central Illinois against the Sangamon Arch. The Sangamon Arch was an east-west structure that probably separated the Grand Tower from the Wapsipinicon Limestone of northern Illinois and Iowa during their deposition. Ithasbeen so modified by post-Devonian move- ments that it is no longer an important structural element. -

Geological and Geophysical Maps of the Illinois Basin–Ozark Dome Region
Illinois Map 23 Geological and Geophysical Maps of the Illinois Basin–Ozark Dome Region Compiled by Stephen Marshak, Timothy H. Larson, and Curtis C. Abert 2016 Prairie Research Institute ILLINOIS STATE GEOLOGICAL SURVEY 615 East Peabody Drive Champaign, Illinois 61820-6918 (217) 244-2414 http://www.isgs.illinois.edu © 2016 University of Illinois Board of Trustees. All rights reserved. For permission information, contact the Illinois State Geological Survey. Introduction Base Maps This map series was initially envisioned as a means to Map 1—Land-Surface Topography provide a visual context for discussions associated with the This map is a digital elevation model at 3× vertical exagger- National Science Foundation EarthScope Workshop entitled ation that displays the present-day ground surface elevation Tectonic Targets for EarthScope in the Mid-continent, held in shaded relief (Figure 1). It shows standard color represen- on April 11–13, 2010, in Urbana, Illinois (Stephen Marshak, tations for elevation. Several key features are prominent on lead convener). Participants were sufficiently interested in the map: the maps, and therefore we decided to publish them. • Topographic manifestations of subsurface structure, The 12 maps in this series form a set that spans the four-state for example, the contrast between (1) the highland of region of Missouri, Illinois, Indiana, and Kentucky. We refer the Ozark Plateau and (2) the lowland of the Illinois to the set as the MIINK series. Although we knew of excel- Basin, (3) the highland associated with the “crustal lent maps in each of these states, few, if any, were available bridge” separating the Reelfoot Rift from (4) the that stitched together information for the entire region. -

Dim Future for Illinois Basin Coal Market Forces and Shifting Preferences Are Eroding Customer Base Domestically and Abroad
Seth Feaster, IEEFA Data Analyst 1 Karl Cates, IEEFA Transition Policy Analyst December 2019 Dim Future for Illinois Basin Coal Market Forces and Shifting Preferences Are Eroding Customer Base Domestically and Abroad Executive Summary Twenty years from now, most of the Illinois Basin coal industry will be gone. Currently one of the major U.S. producing regions of thermal coal for domestic and foreign electricity generation, by 2040 it will have largely faded away as utilities shift to cleaner, cheaper generation resources. In 2018, the Illinois Basin produced 106.8 million tons of thermal coal, about 14 percent of the total mined nationwide. Most of that total was used domestically, the remainder was exported. The Basin, which straddles the Ohio River in parts of three states—southern Illinois, southwest Indiana and western Kentucky—does not produce any of the metallurgical coal used in steelmaking, meaning it cannot escape the rapid transition now under way in the electric generation sector, both domestically and abroad. Domestic utilities, which bought nearly 80 percent of the region’s coal in 2018, have already announced retirement dates for a significant number of plants over this period, as competitive electric markets compel a shift to newer, less expensive and cleaner wind, solar and gas generation, increasingly supported with energy storage. Notably, these retirements include the largest single purchaser of Illinois Basin coal, Duke’s Gibson plant in Indiana, which will close in phases by 2038. Gibson’s closing will not be unique, however. From the beginning of 2019 through 2024, at least 15 U.S. plants that buy Illinois Basin coal—in Alabama, Florida, Georgia, Indiana, Kentucky, North Carolina and Tennessee—will be fully or partially retired.