Isolation and Identification of Fungal Endophytes from Grasses Along the Oregon Coast
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
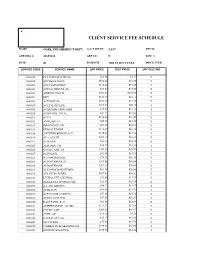
Oakland Sheriff's Dept Fee Schedule 02.09
CLIENT SERVICE FEE SCHEDULE NAME: OAKLAND SHERIFF'S DEPT ACCT SETUP: 2/1/87 REP ID: GRP BILL #: 22857610 GRP CD: N DISC %: FS ID: 01 FS DESCR: 2003 CLIENT FEES PRICE TYPE: SERVICE CODE SERVICE NAME UPF PRICE TEST PRICE UPF DISC IND 0000018 PLT SODIUM CITRATE $12.86 $4.37 Y 0000022 APC RESISTANCE $101.62 $34.55 Y 0000201 ACETAMINOPHEN $110.24 $37.48 Y 0000202 ACETALDEHYDE (B) $73.00 $73.00 N 0000203 ARSENIC,TISSUE $302.00 $302.00 N 0000204 DHT $182.70 $62.12 Y 0000205 ACETONE (B) $102.20 $34.75 Y 0000206 ACETYLCHOLINE $165.00 $56.10 Y 0000208 ACID PHOS, PROS, IMM $35.60 $12.10 Y 0000210 ACID PHOS, TOTAL $37.22 $12.65 Y 0000211 ACTH $110.00 $37.40 Y 0000212 AMYLASE (U) $45.45 $15.45 Y 0000213 IMMUNOFIX, UR $67.39 $22.91 Y 0000214 ETHOSUXIMIDE $112.07 $38.10 Y 0000216 ANTITHROMBIN III ACT $170.46 $57.96 Y 0000219 ALA, QUANT $107.10 $36.41 Y 0000223 ALBUMIN $22.88 $22.88 N 0000224 ALBUMIN, CSF $36.25 $12.33 Y 0000225 CYCLIC AMP, UR $205.80 $69.97 Y 0000227 ALDOLASE $32.08 $10.91 Y 0000228 A-2-MACROGLOB. $78.75 $26.78 Y 0000229 ALDOSTERONE (U) $251.58 $85.54 Y 0000230 ALDOSTERONE $207.10 $70.41 Y 0000231 ALK PHOS ISOENZYMES $61.34 $20.86 Y 0000232 AFP, FLUID W/RFX $107.40 $36.52 Y 0000233 LEUKOCYTE ALK PHOS $34.60 $11.76 Y 0000234 ALKALINE PHOSPHATASE $22.88 $22.88 N 0000235 A-1-ANTITRYPSN $40.22 $13.67 Y 0000236 AMIKACIN $99.91 $33.97 Y 0000237 AFP,TUMOR (CHIRON) $53.86 $18.31 Y 0000238 AMINO ACID SCR $87.15 $29.63 Y 0000240 RAST PANEL #103 $62.00 $62.00 N 0000241 AMPHETAMINE GC/MS $111.70 $37.98 Y 0000242 CYCLIC AMP $205.80 $69.97 Y 0000243 AMYLASE $16.42 $5.58 Y 0000248 HACKBERRY IGE $35.40 $35.40 N 0000249 ANA W/RFX $55.00 $18.70 Y 0000250 *AMIKACIN,PEAK&TROUGH $199.83 $67.94 Y 0000251 ANDROSTENEDIONE $180.65 $61.42 Y 0000252 ANTI-DIUR. -

Paraphaeosphaeria Xanthorrhoeae Fungal Planet Description Sheets 253
252 Persoonia – Volume 38, 2017 Paraphaeosphaeria xanthorrhoeae Fungal Planet description sheets 253 Fungal Planet 560 – 20 June 2017 Paraphaeosphaeria xanthorrhoeae Crous, sp. nov. Etymology. Name refers to Xanthorrhoea, the plant genus from which Notes — The genus Paraconiothyrium (based on P. estuari- this fungus was collected. num) was established by Verkley et al. (2004) to accommodate Classification — Didymosphaeriaceae, Pleosporales, Dothi- several microsphaeropsis-like coelomycetes, some of which deomycetes. had proven abilities to act as biocontrol agents of other fungal pathogens. In a recent study, Verkley et al. (2014) revealed Conidiomata erumpent, globose, pycnidial, brown, 80–150 Paraconiothyrium to be paraphyletic, and separated the genus µm diam, with central ostiole; wall of 3–5 layers of brown tex- from Alloconiothyrium, Dendrothyrium, and Paraphaeosphae- tura angularis. Conidiophores reduced to conidiogenous cells. ria. Paraphaeosphaeria xanthorrhoeae resembles asexual Conidiogenous cells lining the inner cavity, hyaline, smooth, morphs of Paraphaeosphaeria, having pycnidial conidiomata ampulliform, phialidic with periclinal thickening or percurrent with percurrently proliferating conidiogenous cells and aseptate, proliferation at apex, 5–8 × 4–6 µm. Conidia solitary, golden brown, roughened conidia. Phylogenetically, it is distinct from brown, ellipsoid with obtuse ends, thick-walled, roughened, (6–) all taxa presently known to occur in the genus, the closest 7–8(–9) × (3–)3.5 µm. species on ITS being Paraphaeosphaeria sporulosa (GenBank Culture characteristics — Colonies flat, spreading, cover- JX496114; Identities = 564/585 (96 %), 4 gaps (0 %)). ing dish in 2 wk at 25 °C, surface folded, with moderate aerial mycelium and smooth margins. On MEA surface dirty white, reverse luteous. On OA surface dirty white with patches of luteous. -

Molecular Systematics of the Marine Dothideomycetes
available online at www.studiesinmycology.org StudieS in Mycology 64: 155–173. 2009. doi:10.3114/sim.2009.64.09 Molecular systematics of the marine Dothideomycetes S. Suetrong1, 2, C.L. Schoch3, J.W. Spatafora4, J. Kohlmeyer5, B. Volkmann-Kohlmeyer5, J. Sakayaroj2, S. Phongpaichit1, K. Tanaka6, K. Hirayama6 and E.B.G. Jones2* 1Department of Microbiology, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla, 90112, Thailand; 2Bioresources Technology Unit, National Center for Genetic Engineering and Biotechnology (BIOTEC), 113 Thailand Science Park, Paholyothin Road, Khlong 1, Khlong Luang, Pathum Thani, 12120, Thailand; 3National Center for Biothechnology Information, National Library of Medicine, National Institutes of Health, 45 Center Drive, MSC 6510, Bethesda, Maryland 20892-6510, U.S.A.; 4Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon, 97331, U.S.A.; 5Institute of Marine Sciences, University of North Carolina at Chapel Hill, Morehead City, North Carolina 28557, U.S.A.; 6Faculty of Agriculture & Life Sciences, Hirosaki University, Bunkyo-cho 3, Hirosaki, Aomori 036-8561, Japan *Correspondence: E.B. Gareth Jones, [email protected] Abstract: Phylogenetic analyses of four nuclear genes, namely the large and small subunits of the nuclear ribosomal RNA, transcription elongation factor 1-alpha and the second largest RNA polymerase II subunit, established that the ecological group of marine bitunicate ascomycetes has representatives in the orders Capnodiales, Hysteriales, Jahnulales, Mytilinidiales, Patellariales and Pleosporales. Most of the fungi sequenced were intertidal mangrove taxa and belong to members of 12 families in the Pleosporales: Aigialaceae, Didymellaceae, Leptosphaeriaceae, Lenthitheciaceae, Lophiostomataceae, Massarinaceae, Montagnulaceae, Morosphaeriaceae, Phaeosphaeriaceae, Pleosporaceae, Testudinaceae and Trematosphaeriaceae. Two new families are described: Aigialaceae and Morosphaeriaceae, and three new genera proposed: Halomassarina, Morosphaeria and Rimora. -

Endophytic Fungi: Treasure for Anti-Cancerous Compounds
International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 8, Issue 8, 2016 Review Article ENDOPHYTIC FUNGI: TREASURE FOR ANTI-CANCEROUS COMPOUNDS ANAND DILIP FIRODIYAa*, RAJESH KUMAR TENGURIAb aCSRD, Peoples University, Bhopal 462037, Madhya Pradesh, India, bDepartment of Botany, Govt. PG College, Rajgarh 496551, Madhya Pradesh, India Email: [email protected] Received: 22 Apr 2016 Revised and Accepted: 20 June 2016 ABSTRACT Endophytic fungi that live asymptomatically inside the plant tissues have novel bioactive metabolites exhibiting a variety of biological activities, especially against cancer. This review highlights the research progress on the production of anticancer compounds by endophytic fungi from 1990- 2015. Anticancer activity is generally associated with the cytotoxicity of the compounds present in the endophytic fungi. The ubiquitous nature of endophytic fungi synthesise diverse chemicals with promising anticancer activity from either their original host or related species. Modification in fermentation parameters and genetic insight of endophytes may produce novel anti-cancerous compounds. Keywords: Cancer, Medicinal plants, Secondary metabolites © 2016 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/) INTRODUCTION endophytic fungi detectable by high-performance liquid chromate- graphy, nuclear magnetic resonance, mass spectrophotometer and The interest in the biogenic medicines has revived throughout the X-ray crystallography and its cytotoxicity of the bioactive world, as the increase in awareness of the health hazards and compounds against cancer cell lines. The compounds with potential toxicity associated with the random use of synthetic drugs and application were also considered in the selection of antitumor antibiotics [1]. -

AR TICLE Recommended Names for Pleomorphic Genera In
IMA FUNGUS · 6(2): 507–523 (2015) doi:10.5598/imafungus.2015.06.02.14 Recommended names for pleomorphic genera in Dothideomycetes ARTICLE Amy Y. Rossman1, Pedro W. Crous2,3, Kevin D. Hyde4,5, David L. Hawksworth6,7,8, André Aptroot9, Jose L. Bezerra10, Jayarama D. Bhat11, Eric Boehm12, Uwe Braun13, Saranyaphat Boonmee4,5, Erio Camporesi14, Putarak Chomnunti4,5, Dong-Qin Dai4,5, Melvina J. D’souza4,5, Asha Dissanayake4,5,15, E.B. Gareth Jones16, Johannes Z. Groenewald2, Margarita Hernández-Restrepo2,3, Sinang Hongsanan4,5, Walter M. Jaklitsch17, Ruvishika Jayawardena4,5,12, Li Wen Jing4,5, Paul M. Kirk18, James D. Lawrey19, Ausana Mapook4,5, Eric H.C. McKenzie20, Jutamart Monkai4,5, Alan J.L. Phillips21, Rungtiwa Phookamsak4,5, Huzefa A. Raja22, Keith A. Seifert23, Indunil Senanayake4,5, Bernard Slippers3, Satinee Suetrong24, Kazuaki Tanaka25, Joanne E. Taylor26, Kasun M. Thambugala4,5,27, Qing Tian4,5, Saowaluck Tibpromma4,5, Dhanushka N. Wanasinghe4,5,12, Nalin N. Wijayawardene4,5, Saowanee Wikee4,5, Joyce H.C. Woudenberg2, Hai-Xia Wu28,29, Jiye Yan12, Tao Yang2,30, Ying Zhang31 1Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon 97331, USA; corresponding author e-mail: amydianer@ yahoo.com 2CBS-KNAW Fungal Biodiversity Institute, Uppsalalaan 8, 3584 CT Utrecht, The Netherlands 3Department of Microbiology and Plant Pathology, Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria 0002, South Africa 4Center of Excellence in Fungal Research, School of Science, Mae Fah -

And Mushroom-Associated Alkaloids from Two Behavior Modifying
bioRxiv preprint doi: https://doi.org/10.1101/375105; this version posted December 18, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Psychoactive plant- and mushroom-associated alkaloids from two behavior modifying 2 cicada pathogens 3 4 Greg R. Boyce1, Emile Gluck-Thaler2, Jason C. Slot2, Jason E. Stajich3, William J. Davis4, Tim 5 Y. James4, John R. Cooley5, Daniel G. Panaccione1, Jørgen Eilenberg6, Henrik H. De Fine Licht6, 6 Angie M. Macias1, Matthew C. Berger1, Kristen L. Wickert1, Cameron M. Stauder1, Ellie J. 7 Spahr1, Matthew D. Maust1, Amy M. Metheny1, Chris Simon5, Gene Kritsky7, Kathie T. Hodge8, 8 Richard A. Humber8,9, Terry Gullion10, Dylan P. G. Short11, Teiya Kijimoto1, Dan Mozgai12, 9 Nidia Arguedas13, Matt T. Kasson1,* 10 11 1Division of Plant and Soil Sciences, West Virginia University, Morgantown, West Virginia, 12 26506, USA. 13 2Department of Plant Pathology, The Ohio State University, Columbus, Ohio 43210, USA. 14 3Department of Microbiology and Plant Pathology and Institute for Integrative Genome Biology, 15 University of California, Riverside, California 92521, USA. 16 4Department of Ecology and Evolution, University of Michigan, Ann Arbor, Michigan 48109, 17 USA. 18 5Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, 19 Connecticut 06269, USA. 20 6Department of Plant and Environmental Science, University of Copenhagen, Denmark. 21 7Department of Biology, Mount St. Joseph University, Cincinnati, Ohio 45233, USA. 22 8Plant Pathology & Plant-Microbe Biology, School of Integrative Plant Science, Cornell 23 University, Ithaca, New York 14853, USA. -
Fungal Endophytes As Efficient Sources of Plant-Derived Bioactive
microorganisms Review Fungal Endophytes as Efficient Sources of Plant-Derived Bioactive Compounds and Their Prospective Applications in Natural Product Drug Discovery: Insights, Avenues, and Challenges Archana Singh 1,2, Dheeraj K. Singh 3,* , Ravindra N. Kharwar 2,* , James F. White 4,* and Surendra K. Gond 1,* 1 Department of Botany, MMV, Banaras Hindu University, Varanasi 221005, India; [email protected] 2 Department of Botany, Institute of Science, Banaras Hindu University, Varanasi 221005, India 3 Department of Botany, Harish Chandra Post Graduate College, Varanasi 221001, India 4 Department of Plant Biology, Rutgers University, New Brunswick, NJ 08901, USA * Correspondence: [email protected] (D.K.S.); [email protected] (R.N.K.); [email protected] (J.F.W.); [email protected] (S.K.G.) Abstract: Fungal endophytes are well-established sources of biologically active natural compounds with many producing pharmacologically valuable specific plant-derived products. This review details typical plant-derived medicinal compounds of several classes, including alkaloids, coumarins, flavonoids, glycosides, lignans, phenylpropanoids, quinones, saponins, terpenoids, and xanthones that are produced by endophytic fungi. This review covers the studies carried out since the first report of taxol biosynthesis by endophytic Taxomyces andreanae in 1993 up to mid-2020. The article also highlights the prospects of endophyte-dependent biosynthesis of such plant-derived pharma- cologically active compounds and the bottlenecks in the commercialization of this novel approach Citation: Singh, A.; Singh, D.K.; Kharwar, R.N.; White, J.F.; Gond, S.K. in the area of drug discovery. After recent updates in the field of ‘omics’ and ‘one strain many Fungal Endophytes as Efficient compounds’ (OSMAC) approach, fungal endophytes have emerged as strong unconventional source Sources of Plant-Derived Bioactive of such prized products. -

Characterization and Variation of Bacterial and Fungal Communities
bioRxiv preprint doi: https://doi.org/10.1101/2020.10.23.351890; this version posted October 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Characterization and variation of bacterial and fungal communities 2 from the sapwood of Apulian olive varieties with different 3 susceptibility to Xylella fastidiosa 4 5 Arafat Hanani1,2*, Franco Valentini1, Giuseppe Cavallo1, Simona Marianna 6 Sanzani1, Franco Santoro1, Serena Anna Minutillo1, Marilita Gallo1, Maroun 7 El Moujabber1, Anna Maria D'Onghia1, Salvatore Walter Davino2 8 9 1 Department of Integrated pest Management, Mediterranean Agronomic Institute of Bari, Bari, 10 Italy. 11 2Department of Agricultural, Food and Forest Science, University of Palermo, Palermo, Italy. 12 13 *Corresponding author: Arafat Hanani 14 Email: [email protected] 15 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.10.23.351890; this version posted October 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 16 Abstract 17 18 Endophytes are symptomless fungal and/or bacterial microorganisms found in almost all living 19 plant species. The symbiotic association with their host plants by colonizing the internal tissues 20 has endowed them as a valuable tool to suppress diseases, to stimulate growth, and to promote 21 stress resistance. -

Endophytic Fungi of Olive Tree and Their Exploitation in the Biological
ESCUELA SUPERIOR Y TÉCNICA DE INGENIERÍA AGRARIA INGENIERÍA DE BIOSISTEMAS Endophytic fungi of olive tree and their exploitation in the biological control of olive anthracnose Doctoral Thesis Maria de Fátima Tomé Martins Director: Professora Doutora Paula Cristina Santos Baptista León 2020 ESCUELA SUPERIOR Y TÉCNICA DE INGENIERÍA AGRARIA INGENIERÍA DE BIOSISTEMAS Hongos endofíticos del olivo y su aprovechamiento para el control biológico de la antracnosis del olivo Tesis Doctoral Maria de Fátima Tomé Martins Director: Profesora Doctora Paula Cristina Santos Baptista León 2020 This research was supported by FEDER funds through the COMPETE (Operational Programme for Competitiveness Factors) and by the Foundation for Science and Technology (FCT, Portugal) within the POCI-01-0145-FEDER-031133 project and FCT/MCTES to CIMO (UIDB/00690/2020), as well as the Horizon 2020, the European Union’s Framework Programme for Research and Innovation, for financial support the project PRIMA/0002/2018. Fátima Martins also thanks the individual research grant ref. SFRH / BD / 112234/2015 award by FCT. The studies presented in this thesis were performed at the AgroBioTechnology Laboratory, at the Mountain Research Centre (CIMO), School of Agriculture Polytechnic Institute of Bragança. Às minhas filhas Acknowledgements Neste momento, é com enorme gosto e satisfação que agradeço a todos aqueles que direta ou indiretamente contribuíram para a realização e conclusão deste trabalho. Em primeiro lugar gostaria de agradecer em especial à minha orientadora. À Professora Doutora Paula Cristina dos Santos Baptista, da Escola Superior Agrária, por toda a orientação prestada durante a realização do trabalho ao nível laboratorial e escrito, bem como, por todo o conhecimento transmitido, paciência, disponibilidade e acima de tudo pela amizade e carinho demonstrados ao longo destes anos. -

Paraconiothyrium, a New Genus to Accommodate the Mycoparasite Coniothyrium Minitans, Anamorphs of Paraphaeosphaeria, and Four New Species
STUDIES IN MYCOLOGY 50: 323–335. 2004. Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species 1* 2 3 1 Gerard J.M. Verkley , Manuela da Silva , Donald T. Wicklow and Pedro W. Crous 1Centraalbureau voor Schimmelcultures, Fungal Biodiversity Centre, PO Box 85167, NL-3508 AD Utrecht, the Netherlands; 2Fungi Section, Department of Microbiology, INCQS/FIOCRUZ, Av. Brasil, 4365; CEP: 21045-9000, Manguinhos, Rio de Janeiro, RJ, Brazil. 3Mycotoxin Research Unit, National Center for Agricultural Utilization Research, 1815 N. University Street, Peoria, IL 61604, Illinois, U.S.A. *Correspondence: Gerard J.M. Verkley, [email protected] Abstract: Coniothyrium-like coelomycetes are drawing attention as biological control agents, potential bioremediators, and producers of antibiotics. Four genera are currently used to classify such anamorphs, namely, Coniothyrium, Microsphaeropsis, Cyclothyrium, and Cytoplea. The morphological plasticity of these fungi, however, makes it difficult to ascertain their best generic disposition in many cases. A new genus, Paraconiothyrium is here proposed to accommodate four new species, P. estuarinum, P. brasiliense, P. cyclothyrioides, and P. fungicola. Their formal descriptions are based on anamorphic characters as seen in vitro. The teleomorphs of these species are unknown, but maximum parsimony analysis of ITS and partial SSU nrDNA sequences showed that they belong in the Pleosporales and group in a clade including Paraphaeosphaeria s. str., the biocontrol agent Coniothyrium minitans, and the ubiquitous soil fungus Coniothyrium sporulosum. Coniothyrium minitans and C. sporulosum are therefore also combined into the genus Paraconiothyrium. The anamorphs of Paraphaeosphaeria michotii and Paraphaeosphaeria pilleata are regarded representative of Paraconiothyrium, but remain formally unnamed. -

Fungal Endophytes: Promising Tools for Pharmaceutical Science
Int. J. Pharm. Sci. Rev. Res., 25(2), Mar – Apr 2014; Article No. 25, Pages: 128-138 ISSN 0976 – 044X Research Article Fungal Endophytes: Promising Tools for Pharmaceutical Science Pramod Kumar Pandey1,2, Siddhartha Singh1, Raj Naraian Singh Yadav2, Amit Kumar Singh1*, M. Chandra Kumar Singh1 1Department of Basic Science and Humanities, College of Horticulture and Forestry, Central Agricultural University, Pasighat, Arunachal Pradesh, India. 2Centre for Studies in Biotechnology, Dibrugarh University, Dibrugarh, Assam, India. *Corresponding author’s E-mail: [email protected] Accepted on: 27-01-2014; Finalized on: 31-03-2014. ABSTRACT Fungal endophytes are microorganisms that internally infect living plant tissues without causing any visible symptom of infection, and live in mutualistic relationship with plants for at least a part of their life cycle. Every plant in the world is reservoir of one or more number of endophytes. In recent year’s special attention have been made towards endophytic fungi because of their ability to synthesize several novel bioactive compounds not previously known to biological system which are important for pharmaceutical, agricultural and industrial sector. This review describes information on endophyte diversity, as well as production of secondary metabolites with special emphasis on anti cancerous, antimicrobial, antiviral, antibiotics along with huge number of other secondary metabolites for commercial exploitation in pharmaceutical and medical field. Furthermore, the chemical potential of endophytic fungi for drug discovery will be discussed with focus on the detection of pharmaceutically valuable plant constituents as products of fungal biosynthesis in recent years. At present a huge world population is suffering from the problem caused by drug resistant microbes (bacteria, parasitic protozoans and fungus) which decreases the efficiency of synthetic drugs. -

COMPARATIVE BIOLOGICAL EVALUATION of SIX ENDOPHYTIC FUNGI ISOLATED from VINCA ROSEA LEAVES Ahmed M
Az. J. Pharm Sci. Vol. 59, March, 2019. 137 COMPARATIVE BIOLOGICAL EVALUATION OF SIX ENDOPHYTIC FUNGI ISOLATED FROM VINCA ROSEA LEAVES Ahmed M. Metwaly*1, Mohamed A. Ashour1, Shabana Khan2, Guoyi Ma2, Hazem A. Kadry1, Atef A. El-Hela1, Abd-Elsalam I. Mohammad1, Stephen J. Cutler3 and Samir A. Ross2 1Department of Pharmacognosy, Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt 2 Department of Biomolecular Science, National Center for Natural Products Research, University of Mississippi, Oxford, MS 38677, USA 3Department of Medicinal Chemistry, School of Pharmacy, the University of Mississippi, Oxford, MS 38677, USA *Corresponding authors: [email protected] Abstract In this study, a total of six endophytic fungi have been isolated from Vinca rosea (Apocynaceae) leaves growing in Egypt. The isolated fungi were identified morphologically and microscopically up to species to be; Alternaria phragmospora, Aspergillus awamori, Penicillium duclauxii, Penicillium melinii, Nigrospora sphaerica and Mucor ramosissimus. The extracts of the all identified fungi were screened biologically for antileukemic, cytotoxic, antimalarial, antileishmanial, antimicrobial, antioxidant and anti-inflammatory activities as well as for cannabinoid and opioid receptor binding affinities. Out of the six examined fungal extracts four exhibited promising antimalarial activities, four showed modeate antileukemic activities and two of them exhibited cytotoxic activities, three showed antioxidant activities, three exhibited anti-inflammatory activities, and the only one showed an antifungal activity. Alternaria phragmospora was the most active antimalarial agent inhibiting Plasmodium falciparum D6 and W2 clones with IC50 values of 1.9 and 2.1 μg/mL, respectively. Alternaria phragmospora extract was subjected to liquid-liquid partition using 90% MeOH, hexane then BuOH and H2O.