Endophytic Fungi: Treasure for Anti-Cancerous Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
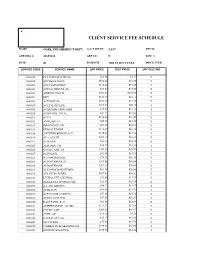
Oakland Sheriff's Dept Fee Schedule 02.09
CLIENT SERVICE FEE SCHEDULE NAME: OAKLAND SHERIFF'S DEPT ACCT SETUP: 2/1/87 REP ID: GRP BILL #: 22857610 GRP CD: N DISC %: FS ID: 01 FS DESCR: 2003 CLIENT FEES PRICE TYPE: SERVICE CODE SERVICE NAME UPF PRICE TEST PRICE UPF DISC IND 0000018 PLT SODIUM CITRATE $12.86 $4.37 Y 0000022 APC RESISTANCE $101.62 $34.55 Y 0000201 ACETAMINOPHEN $110.24 $37.48 Y 0000202 ACETALDEHYDE (B) $73.00 $73.00 N 0000203 ARSENIC,TISSUE $302.00 $302.00 N 0000204 DHT $182.70 $62.12 Y 0000205 ACETONE (B) $102.20 $34.75 Y 0000206 ACETYLCHOLINE $165.00 $56.10 Y 0000208 ACID PHOS, PROS, IMM $35.60 $12.10 Y 0000210 ACID PHOS, TOTAL $37.22 $12.65 Y 0000211 ACTH $110.00 $37.40 Y 0000212 AMYLASE (U) $45.45 $15.45 Y 0000213 IMMUNOFIX, UR $67.39 $22.91 Y 0000214 ETHOSUXIMIDE $112.07 $38.10 Y 0000216 ANTITHROMBIN III ACT $170.46 $57.96 Y 0000219 ALA, QUANT $107.10 $36.41 Y 0000223 ALBUMIN $22.88 $22.88 N 0000224 ALBUMIN, CSF $36.25 $12.33 Y 0000225 CYCLIC AMP, UR $205.80 $69.97 Y 0000227 ALDOLASE $32.08 $10.91 Y 0000228 A-2-MACROGLOB. $78.75 $26.78 Y 0000229 ALDOSTERONE (U) $251.58 $85.54 Y 0000230 ALDOSTERONE $207.10 $70.41 Y 0000231 ALK PHOS ISOENZYMES $61.34 $20.86 Y 0000232 AFP, FLUID W/RFX $107.40 $36.52 Y 0000233 LEUKOCYTE ALK PHOS $34.60 $11.76 Y 0000234 ALKALINE PHOSPHATASE $22.88 $22.88 N 0000235 A-1-ANTITRYPSN $40.22 $13.67 Y 0000236 AMIKACIN $99.91 $33.97 Y 0000237 AFP,TUMOR (CHIRON) $53.86 $18.31 Y 0000238 AMINO ACID SCR $87.15 $29.63 Y 0000240 RAST PANEL #103 $62.00 $62.00 N 0000241 AMPHETAMINE GC/MS $111.70 $37.98 Y 0000242 CYCLIC AMP $205.80 $69.97 Y 0000243 AMYLASE $16.42 $5.58 Y 0000248 HACKBERRY IGE $35.40 $35.40 N 0000249 ANA W/RFX $55.00 $18.70 Y 0000250 *AMIKACIN,PEAK&TROUGH $199.83 $67.94 Y 0000251 ANDROSTENEDIONE $180.65 $61.42 Y 0000252 ANTI-DIUR. -

Hallucinogens: a Cause of Convulsive Ergot Psychoses
Loma Linda University TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works Loma Linda University Electronic Theses, Dissertations & Projects 6-1976 Hallucinogens: a Cause of Convulsive Ergot Psychoses Sylvia Dahl Winters Follow this and additional works at: https://scholarsrepository.llu.edu/etd Part of the Psychiatry Commons Recommended Citation Winters, Sylvia Dahl, "Hallucinogens: a Cause of Convulsive Ergot Psychoses" (1976). Loma Linda University Electronic Theses, Dissertations & Projects. 976. https://scholarsrepository.llu.edu/etd/976 This Thesis is brought to you for free and open access by TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works. It has been accepted for inclusion in Loma Linda University Electronic Theses, Dissertations & Projects by an authorized administrator of TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works. For more information, please contact [email protected]. ABSTRACT HALLUCINOGENS: A CAUSE OF CONVULSIVE ERGOT PSYCHOSES By Sylvia Dahl Winters Ergotism with vasoconstriction and gangrene has been reported through the centuries. Less well publicized are the cases of psychoses associated with convulsive ergotism. Lysergic acid amide a powerful hallucinogen having one.-tenth the hallucinogenic activity of LSD-25 is produced by natural sources. This article attempts to show that convulsive ergot psychoses are mixed psychoses caused by lysergic acid amide or similar hallucinogens combined with nervous system -

And Mushroom-Associated Alkaloids from Two Behavior Modifying
bioRxiv preprint doi: https://doi.org/10.1101/375105; this version posted December 18, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Psychoactive plant- and mushroom-associated alkaloids from two behavior modifying 2 cicada pathogens 3 4 Greg R. Boyce1, Emile Gluck-Thaler2, Jason C. Slot2, Jason E. Stajich3, William J. Davis4, Tim 5 Y. James4, John R. Cooley5, Daniel G. Panaccione1, Jørgen Eilenberg6, Henrik H. De Fine Licht6, 6 Angie M. Macias1, Matthew C. Berger1, Kristen L. Wickert1, Cameron M. Stauder1, Ellie J. 7 Spahr1, Matthew D. Maust1, Amy M. Metheny1, Chris Simon5, Gene Kritsky7, Kathie T. Hodge8, 8 Richard A. Humber8,9, Terry Gullion10, Dylan P. G. Short11, Teiya Kijimoto1, Dan Mozgai12, 9 Nidia Arguedas13, Matt T. Kasson1,* 10 11 1Division of Plant and Soil Sciences, West Virginia University, Morgantown, West Virginia, 12 26506, USA. 13 2Department of Plant Pathology, The Ohio State University, Columbus, Ohio 43210, USA. 14 3Department of Microbiology and Plant Pathology and Institute for Integrative Genome Biology, 15 University of California, Riverside, California 92521, USA. 16 4Department of Ecology and Evolution, University of Michigan, Ann Arbor, Michigan 48109, 17 USA. 18 5Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, 19 Connecticut 06269, USA. 20 6Department of Plant and Environmental Science, University of Copenhagen, Denmark. 21 7Department of Biology, Mount St. Joseph University, Cincinnati, Ohio 45233, USA. 22 8Plant Pathology & Plant-Microbe Biology, School of Integrative Plant Science, Cornell 23 University, Ithaca, New York 14853, USA. -

Spatial Patterns of Claviceps Purpurea in Kentucky Bluegrass And
SPATIAL PATTERNS OF CLAVICEPS PURPUREA IN KENTUCKY BLUEGRASS AND PERENNIAL RYEGRASS GROWN FOR SEED AND EFFECT OF SOIL-APPLIED FUNGICIDES ON GERMINATION OF ERGOT SCLEROTIA J.K.S. Dung, D.L. Walenta, S.C. Alderman, and P.B. Hamm Introduction protective fungicide applications are required during Ergot, caused by the fungus Claviceps purpurea, is anthesis. Some growers experience severe ergot an important floral disease of grasses that issues even after four fungicide applications. A significantly impacts Kentucky bluegrass (KBG) and limited number of fungicides are currently available perennial ryegrass (PRG) seed production in the and new products or application strategies need to be Pacific Northwest (PNW). The fungus infects evaluated. In addition to timing fungicides during unfertilized ovaries and replaces seed with elongated anthesis, when flowers are susceptible to infection, it black fungal bodies called sclerotia, which may be possible to apply fungicides to sclerotia in overwinter in the soil and germinate to produce the field during the fall and/or as they begin to airborne ascospores in the spring. In addition to germinate in the spring before they release spores. yield losses from ergot, the repeated cleanings Soil-applied fungicides may reduce sclerotia required to remove ergot sclerotia to achieve seed germination and spore production, limiting the certification standards result in additional seed loss production of primary inoculum and subsequent as well as extra costs in time and labor. ergot infection (Hardison 1975). Although ergot is a persistent problem in PNW grass The objectives of this study were to: 1) quantify and seed production, the incidence and intensity of ergot describe the spatial patterns of ergot epidemics in epidemics can vary regionally, locally, and from commercial KBG and PRG fields in Oregon and year to year. -

Phomopsis Seed Decay of Soybean
13 Phomopsis Seed Decay of Soybean Shuxian Li United States Department of Agriculture-Agricultural Research Service Crop Genetics Research Unit, Stoneville, MS 38776 USA 1. Introduction Phomopsis seed decay (PSD) of soybean, Glycine max (L.) Merrill, is the major cause of poor seed quality in most soybean-growing countries (Sinclair, 1993). The disease is caused primarily by the fungal pathogen, Phomopsis longicolla, along with other Phomopsis and Diaporthe spp. PSD severely affects soybean seed quality due to reduction in seed viability and oil content, alteration of seed composition, and increased frequencies of moldy and/or split beans (Hepperly & Sinclair, 1978; Rupe and Ferriss, 1986; Rupe 1990; Wrather at al., 2004). Hot and humid environmental conditions, especially during the period from the pod fill through harvest stages, favor pathogen growth and disease development (Balducchi & McGee, 1987; Hartman et al., 1999). PSD has resulted in significant economic losses (Baird et al., 2001; Hepperly and Sinclair, 1978). Losses on a worldwide basis were approximately 0.19 million metric tons (MMT) in 1994 (Kulik & Sinclair, 1999). Effects of PSD on yields in the United States from 1996 to 2007 ranged from 0.38 to 0.43 MMT (Wrather & Koenning, 2009). In 2009, due to the prevalence of hot and humid environmental conditions from pod fill to harvest in the southern United States, PSD caused over 12 million bushels of yield losses in 16 states (Koenning, 2010). 2. Disease symptoms Soybean seeds infected by P. longicolla or other Phomopsis spp. range from symptomless to shriveled, elongated, or cracked, and often appear chalky-white (Fig. 1). -
Fungal Endophytes As Efficient Sources of Plant-Derived Bioactive
microorganisms Review Fungal Endophytes as Efficient Sources of Plant-Derived Bioactive Compounds and Their Prospective Applications in Natural Product Drug Discovery: Insights, Avenues, and Challenges Archana Singh 1,2, Dheeraj K. Singh 3,* , Ravindra N. Kharwar 2,* , James F. White 4,* and Surendra K. Gond 1,* 1 Department of Botany, MMV, Banaras Hindu University, Varanasi 221005, India; [email protected] 2 Department of Botany, Institute of Science, Banaras Hindu University, Varanasi 221005, India 3 Department of Botany, Harish Chandra Post Graduate College, Varanasi 221001, India 4 Department of Plant Biology, Rutgers University, New Brunswick, NJ 08901, USA * Correspondence: [email protected] (D.K.S.); [email protected] (R.N.K.); [email protected] (J.F.W.); [email protected] (S.K.G.) Abstract: Fungal endophytes are well-established sources of biologically active natural compounds with many producing pharmacologically valuable specific plant-derived products. This review details typical plant-derived medicinal compounds of several classes, including alkaloids, coumarins, flavonoids, glycosides, lignans, phenylpropanoids, quinones, saponins, terpenoids, and xanthones that are produced by endophytic fungi. This review covers the studies carried out since the first report of taxol biosynthesis by endophytic Taxomyces andreanae in 1993 up to mid-2020. The article also highlights the prospects of endophyte-dependent biosynthesis of such plant-derived pharma- cologically active compounds and the bottlenecks in the commercialization of this novel approach Citation: Singh, A.; Singh, D.K.; Kharwar, R.N.; White, J.F.; Gond, S.K. in the area of drug discovery. After recent updates in the field of ‘omics’ and ‘one strain many Fungal Endophytes as Efficient compounds’ (OSMAC) approach, fungal endophytes have emerged as strong unconventional source Sources of Plant-Derived Bioactive of such prized products. -

Diversification of Ergot Alkaloids in Natural and Modified Fungi
Toxins 2015, 7, 201-218; doi:10.3390/toxins7010201 OPEN ACCESS toxins ISSN 2072-6651 www.mdpi.com/journal/toxins Review Diversification of Ergot Alkaloids in Natural and Modified Fungi Sarah L. Robinson and Daniel G. Panaccione * Division of Plant and Soil Sciences, West Virginia University, Morgantown, WV 26506, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-304-293-8819; Fax: +1-304-293-2960. Academic Editor: Christopher L. Schardl Received: 21 November 2014 / Accepted: 14 January 2015 / Published: 20 January 2015 Abstract: Several fungi in two different families––the Clavicipitaceae and the Trichocomaceae––produce different profiles of ergot alkaloids, many of which are important in agriculture and medicine. All ergot alkaloid producers share early steps before their pathways diverge to produce different end products. EasA, an oxidoreductase of the old yellow enzyme class, has alternate activities in different fungi resulting in branching of the pathway. Enzymes beyond the branch point differ among lineages. In the Clavicipitaceae, diversity is generated by the presence or absence and activities of lysergyl peptide synthetases, which interact to make lysergic acid amides and ergopeptines. The range of ergopeptines in a fungus may be controlled by the presence of multiple peptide synthetases as well as by the specificity of individual peptide synthetase domains. In the Trichocomaceae, diversity is generated by the presence or absence of the prenyl transferase encoded by easL (also called fgaPT1). Moreover, relaxed specificity of EasL appears to contribute to ergot alkaloid diversification. The profile of ergot alkaloids observed within a fungus also is affected by a delayed flux of intermediates through the pathway, which results in an accumulation of intermediates or early pathway byproducts to concentrations comparable to that of the pathway end product. -

Ergometrine Maleate
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines Evaluation Unit EMEA/MRL/237/97-FINAL June 1999 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS ERGOMETRINE MALEATE SUMMARY REPORT 1. Ergometrine is a naturally occurring alkaloid found in ergot (Claviceps purpurea). It is classified as a water-soluble lysergic acid derivative, and is an orally-active stimulant of uterine contractions. The maleate salt (ergometrine maleate) exhibits greater stability than the free base and is the usual form in which the alkaloid is used in medicinal products. It is used in veterinary medicine in the control of postpartum uterine haemorrhage, removal of fluid from atonic uteri, to prevent pro-lapsed uteri, and judiciously in terms of timing to aid in suturing the uterus after caesarean section or in replacing an everted uterus. Dose regimens are: cows and mares: 2 to 5 mg/animal (intravenously or intramuscularly); ewes, goats and sows: 0.5 to 1 mg/animal (intramuscularly). In human medicine, it is used orally and parenterally in the prevention and treatment of postpartum haemorrhage caused by uterine atony and for the stimulation of uterine involution. Usual oral doses are 500 µg 3 times daily up to 1.8 mg daily (approximately 0.03 mg/kg bw). Ergot alkaloids have been reported to be present in flour from rye, wheat and barley in amounts ranging from 0.01 to 2.36 mg/kg flour. EU legislation restricts the maximum percentage of ergot tolerated in flour to 0.1%. Total daily human intake of ergot alkaloids from contaminated foodstuffs of plant origin has been estimated as up to 7.8 µg/person. -

The History of Ergot Of
J R Coll Physicians Edinb 2009; 39:365–9 Paper doi:10.4997/JRCPE.2009.416 © 2009 Royal College of Physicians of Edinburgh The history of ergot of rye (Claviceps purpurea) II: 1900–1940 MR Lee Emeritus Professor of Clinical Pharmacology and Therapeutics, University of Edinburgh, Edinburgh, UK ABSTRACT Ergot, in 1900, was a ‘chemical mess’. Henry Wellcome, the Published online December 2009 pharmaceutical manufacturer, invited Henry Hallett Dale, a physiologist, to join his research department and solve this problem. Dale, in turn, recruited an Correspondence to MR Lee, outstanding group of scientists, including George Barger, Arthur Ewins and 112 Polwarth Terrace, Harold Dudley, who would make distinguished contributions not only to the Edinburgh EH11 1NN chemistry of ergot but also to the identification of acetylcholine, histamine and tel. +44 (0)131 337 7386 tyramine and to studies on their physiological effects. Initially Barger and Dale isolated the compound ergotoxine, but this proved to be a false lead; it was later shown to be a mixture of three different ergot alkaloids. The major success of the Wellcome group was the discovery and isolation of ergometrine, which would prove to be life-saving in postpartum haemorrhage. In 1917 Arthur Stoll and his colleagues started work on ergot at Sandoz Pharmaceuticals in Basel. A series of important results emerged over the next 30 years, including the isolation of ergotamine in 1918, an effective treatment for migraine with aura. KEYWORDS Henry Dale, ergometrine, ergotamine, ergotoxine, migraine, postpartum haemorrhage DECLaratION OF INTERESTS No conflict of interests declared. In 1904, at the age of 29, Henry Hallett Dale joined the Burroughs Wellcome Research Laboratory in London (Figure 1). -

Mini Review on Psychedelic Drugs: Illumination on the Hidden Aspects of Mind
Review Article Mini Review on Psychedelic Drugs: Illumination on the Hidden Aspects of Mind Lemlem Hussien Salih and Atul Kaushik* Department of Medicinal Chemistry, School of Pharmacy, Asmara College of Health Sciences, Asmara, Eritrea ABSTRACT Psychedelics constitute a class of psychoactive drugs with unique effects on consciousness. Psychedelic means mind/soul "revealing" and refers to the ability of these drugs to illuminate normally hidden aspects of mind or psyche. Many psychedelic agents occur in nature; others are synthetically produced. Naturally occurring psychedelic drugs have been inhaled, ingested, worshiped, and reviled since Address for prehistory. The phenomenology of the hallucinogenic experience is Correspondence extremely complex, sensory, emotional, cognitive, and spiritual, levels. Most psychedelic drugs structurally resemble with Department of neurotransmitters: acetylcholine, two catecholamines (nor Medicinal Chemistry, epinephrine and dopamine), and serotonin. These structural School of Pharmacy, similarities lead to three classes for categorizing psychedelic drugs: Asmara College of anticholinergic, catecholamine-like, and serotonin-like. And also a Health Sciences, fourth class of psychedelic drugs can be included, the psychedelic Asmara, Eritrea anesthetics. This mini review focuses on pharmacological and Tel.- +291-1-186041 medicinal aspects of this class. E-mail: atul_kaushik29 Keywords: Psychedelic drugs, Pharmacological features of @rediffmail.com psychedelic drugs, SAR of psychedelic drugs. INTRODUCTION "Rational consciousness...is but one produced. Naturally occurring psychedelic special type of consciousness, whilst all drugs have been inhaled, ingested, about it, parted from it by the filmiest of worshiped, and reviled since prehistory. screens; there lie potential forms of Native American shamans consumed consciousness entirely different."-William psychedelic plants such as the peyote cactus James. -

Fungal Endophytes: Promising Tools for Pharmaceutical Science
Int. J. Pharm. Sci. Rev. Res., 25(2), Mar – Apr 2014; Article No. 25, Pages: 128-138 ISSN 0976 – 044X Research Article Fungal Endophytes: Promising Tools for Pharmaceutical Science Pramod Kumar Pandey1,2, Siddhartha Singh1, Raj Naraian Singh Yadav2, Amit Kumar Singh1*, M. Chandra Kumar Singh1 1Department of Basic Science and Humanities, College of Horticulture and Forestry, Central Agricultural University, Pasighat, Arunachal Pradesh, India. 2Centre for Studies in Biotechnology, Dibrugarh University, Dibrugarh, Assam, India. *Corresponding author’s E-mail: [email protected] Accepted on: 27-01-2014; Finalized on: 31-03-2014. ABSTRACT Fungal endophytes are microorganisms that internally infect living plant tissues without causing any visible symptom of infection, and live in mutualistic relationship with plants for at least a part of their life cycle. Every plant in the world is reservoir of one or more number of endophytes. In recent year’s special attention have been made towards endophytic fungi because of their ability to synthesize several novel bioactive compounds not previously known to biological system which are important for pharmaceutical, agricultural and industrial sector. This review describes information on endophyte diversity, as well as production of secondary metabolites with special emphasis on anti cancerous, antimicrobial, antiviral, antibiotics along with huge number of other secondary metabolites for commercial exploitation in pharmaceutical and medical field. Furthermore, the chemical potential of endophytic fungi for drug discovery will be discussed with focus on the detection of pharmaceutically valuable plant constituents as products of fungal biosynthesis in recent years. At present a huge world population is suffering from the problem caused by drug resistant microbes (bacteria, parasitic protozoans and fungus) which decreases the efficiency of synthetic drugs. -

COMPARATIVE BIOLOGICAL EVALUATION of SIX ENDOPHYTIC FUNGI ISOLATED from VINCA ROSEA LEAVES Ahmed M
Az. J. Pharm Sci. Vol. 59, March, 2019. 137 COMPARATIVE BIOLOGICAL EVALUATION OF SIX ENDOPHYTIC FUNGI ISOLATED FROM VINCA ROSEA LEAVES Ahmed M. Metwaly*1, Mohamed A. Ashour1, Shabana Khan2, Guoyi Ma2, Hazem A. Kadry1, Atef A. El-Hela1, Abd-Elsalam I. Mohammad1, Stephen J. Cutler3 and Samir A. Ross2 1Department of Pharmacognosy, Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt 2 Department of Biomolecular Science, National Center for Natural Products Research, University of Mississippi, Oxford, MS 38677, USA 3Department of Medicinal Chemistry, School of Pharmacy, the University of Mississippi, Oxford, MS 38677, USA *Corresponding authors: [email protected] Abstract In this study, a total of six endophytic fungi have been isolated from Vinca rosea (Apocynaceae) leaves growing in Egypt. The isolated fungi were identified morphologically and microscopically up to species to be; Alternaria phragmospora, Aspergillus awamori, Penicillium duclauxii, Penicillium melinii, Nigrospora sphaerica and Mucor ramosissimus. The extracts of the all identified fungi were screened biologically for antileukemic, cytotoxic, antimalarial, antileishmanial, antimicrobial, antioxidant and anti-inflammatory activities as well as for cannabinoid and opioid receptor binding affinities. Out of the six examined fungal extracts four exhibited promising antimalarial activities, four showed modeate antileukemic activities and two of them exhibited cytotoxic activities, three showed antioxidant activities, three exhibited anti-inflammatory activities, and the only one showed an antifungal activity. Alternaria phragmospora was the most active antimalarial agent inhibiting Plasmodium falciparum D6 and W2 clones with IC50 values of 1.9 and 2.1 μg/mL, respectively. Alternaria phragmospora extract was subjected to liquid-liquid partition using 90% MeOH, hexane then BuOH and H2O.