Taking Plant Pharmaceuticals from Research to Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Prespective Study of Clitoria Ternatia and Its Pharmacological Importance
High Technology Letters ISSN NO : 1006-6748 A PRESPECTIVE STUDY OF CLITORIA TERNATIA AND ITS PHARMACOLOGICAL IMPORTANCE S. GEJALAKSHMI *1, N. HARIKRISHNAN FACULTY OF PHARMACY, DR.M.G.R. EDUCATIONAL AND RESEARCH INSTITUTE, VELAPPANCHAVADI, CHENNAI-77 Abstract: Medicinal herbs and aromatic plants have been extensively used for the past few decades due to its potency and minimal side effects. By observing the medicinal importance of the climbing herb Clitoria ternatea (CT)of Fabeacea family and commonly known as Butterfly pea and Shankpushpi has been taken up due to its high medicinal value due to its wide range of use over decade as memory enhancer,antidepressant,anticonvulsant,transquilizers and sedative agent.A series of secondary metabolite including triterpenoids,flavone glycosides,anthocyanins and i steroids has been isolated from CT extracts.CT plant has wide range of pharmacological activity such as antimicrobial,antipyretic,diuretic,local anaesthetic.CT has been used for several diseases due to availability of several active constituents like alkaloids,flavanoids,saponins,tannins,carbohydrates .This review is an platform to explore the phytochemical investigation and pharmacological importance of CT,which have been practiced in traditional system of medicine and its future potential prespectives in view of innumerable therapeutic importance on this well-known twinning climber. Key words: Shankpushpi,phytochemical,antibacterial,anti- fungal, anti-cancer Introduction: Herbal drugs has an impact for curing disorders. The medicinal herbs are rich in various phytochemical constituents which has been found for traditional system of medicines. In the present reveiw focused on the traditional importance of clitoria ternatea. (CT).It is perennial twinning herb. It is a member of fabiaecea family and it has various synonym like blue pea. -

Butterfly Pea (Clitoria Ternatea) | Feedipedia
Butterfly pea (Clitoria ternatea) | Feedipedia Animal feed resources Feedipedia information system Home About Feedipedia Team Partners Get involved Contact us Butterfly pea (Clitoria ternatea) Automatic translation Description Nutritional aspects Nutritional tables References Sélectionner une langue ▼ Click on the "Nutritional aspects" tab for recommendations for ruminants, pigs, poultry, rabbits, horses, fish and crustaceans Feed categories All feeds Forage plants Cereal and grass forages Legume forages Forage trees Aquatic plants Common names Other forage plants Plant products/by-products Butterfly pea, blue pea, kordofan pea, cordofan pea, Asian pigeonwings [English]; pois bleu [French]; clitoria azul, azulejo, Cereal grains and by-products papito, zapatico de la reina, zapotillo, conchita azul, campanilla, bandera, choroque, lupita, pito de parra, bejuco de conchitas Legume seeds and by-products [Spanish]; cunhã, Fula criqua [Portuguese]; kittelbloem [Dutch]; Blaue Klitorie [German]; tembang telang [Indonesian]; Bunga Oil plants and by-products telang [Malay]; Mavi Kelebek Sarmaşığı [Turkish]; Chi Đậu biếc [Vietnamese]; [Bengali]; 蝶豆 [Chinese]; Fruits and by-products [Hindi]; [Malayalam]; [Marathi]; [Tamul]; [Telugu]; Roots, tubers and by-products ดอกอญชั นั [Thai] Sugar processing by-products Plant oils and fats Species Other plant by-products Feeds of animal origin Clitoria ternatea L. [Fabaceae] Animal by-products Dairy products/by-products Synonyms Animal fats and oils Insects Clitoria albiflora Mattei; Clitoria bracteata Poir.; Clitoria mearnsii De Wild.; Clitoria tanganicensis Micheli; Clitoria zanzibarensis Other feeds Vatke Minerals Other products Feed categories Legume forages Legume seeds and by-products Forage plants Latin names Plant and animal families Related feed(s) Plant and animal species Description Resources The butterfly pea (Clitoria ternatea L.) is a vigorous, trailing, scrambling or climbing tropical legume. -

Evolvulus Alsinoides (Convolvulaceae): an American Herb in the Old World Daniel F
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Available online at www.sciencedirect.com Journal of Ethnopharmacology 117 (2008) 185–198 Review Evolvulus alsinoides (Convolvulaceae): An American herb in the Old World Daniel F. Austin Arizona-Sonora Desert Museum, 2021 North Kinney Road, Tucson, AZ 85743, USA Received 23 October 2007; received in revised form 28 January 2008; accepted 29 January 2008 Available online 12 February 2008 Abstract People in the Indian region often apply shankhapushpi and vishnukranti, two Sanskrit-based common names, to Evolvulus alsinoides. These are pre-European names that are applied to a medicinal American species transported into the area. The period of introduction is uncertain, but probably took place in the 1500s or 1600s. Examination of relationships of Evolvulus alsinoides, geographic distribution, its names in Asia, medical uses, and chemical and laboratory analysis indicates that the alien plant was adopted, given an ancient Indian name, and incorporated into some Old World pharmacopoeias. The herb apparently was included in medicines because it not only reminded people of certain aspects of their gods and goddesses, but also because the chemicals it contained were useful against some maladies. -

Healthy Food Traditions of Asia: Exploratory Case Studies From
Harmayani et al. Journal of Ethnic Foods (2019) 6:1 Journal of Ethnic Foods https://doi.org/10.1186/s42779-019-0002-x ORIGINALARTICLE Open Access Healthy food traditions of Asia: exploratory case studies from Indonesia, Thailand, Malaysia, and Nepal Eni Harmayani1, Anil Kumar Anal2, Santad Wichienchot3, Rajeev Bhat4, Murdijati Gardjito1, Umar Santoso1, Sunisa Siripongvutikorn5, Jindaporn Puripaatanavong6 and Unnikrishnan Payyappallimana7* Abstract Asia represents rich traditional dietary diversity. The rapid diet transition in the region is leading to a high prevalence of non-communicable diseases. The aim of this exploratory study was to document traditional foods and beverages and associated traditional knowledge that have potential positive health impacts, from selected countries in the region. The study also focused on identifying their importance in the prevention and management of lifestyle-related diseases and nutritional deficiencies as well as for the improvement of the overall health and wellbeing. This was conducted in selected locations in Indonesia, Thailand, Malaysia and Nepal through a qualitative method with a pre-tested documentation format. Through a detailed documentation of their health benefits, the study tries to highlight the significance of traditional foods in public health as well as their relevance to local market economies towards sustainable production and consumption and sustainable community livelihoods. Keywords: Traditional foods, Ethnic recipes, Asian health food traditions, Cultural dietary diversity, Indonesia, Thailand, Malaysia and Nepal Introduction Due to the dynamic adaptations to local biocultural con- Asia represents vast geographic, socioeconomic, bio- texts and refinement over generations through empirical logical, and cultural diversity. This is also reflected in the observations, they assume to have positive health impacts dietary diversity of traditional foods. -
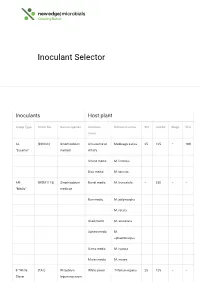
Inoculant Selector
Inoculant Selector Inoculants Host plant Group Type Strain No. Genus/species Common Botanical name Std Jumbo Mega Vial name AL (RRI128) Sinorhizobium All Lucerne or Medicago sativa 25 125 – 100 “Lucerne” meliloti Alfalfa Strand medic M. littoralis Disc medic M. tornata AM (WSM1115) Sinorhizobium Barrel medic M. truncatula – 250 – – “Medic” medicae Burr medic M. polymorpha M. rotata Snail medic M. scutellata Sphere medic M. sphaerocarpus Gama medic M. rugosa Murex medic M. murex B “White (TA1) Rhizobium White clover Trifolium repens 25 125 – – Clover leguminosarum bv. trifolii Red clover T. pratense Strawberry T. fragiferum clover Alsike clover T. hybridum Berseem, T. alexandrinum Egyptian clover Cluster, Ball T. glomeratum clover Suckling T. dubium clover C “Sub (WSM1325) Rhizobium Crimson Trifolium 50 250 – 200 Clover” leguminosarum clover incarnatum bv. trifolii Cupped clover T. cheleri Helmet clover T. clypeatum Rose clover T. hirtum Subterranean T. subterraneum clover var.subterraneum Subterranean T. subterraneum clover var.yanninicum Subterranean T. subterraneum clover var.brachy calycinum Arrowleaf T. vesiculosum 25 125 – 100 clover Balansa T. michelianum clover Bladder clover T. spumosum Gland clover T. glanduliferum Purple clover T. purpureum Persian clover T. resupinatum F “Faba & (WSM1455) Rhizobium Faba, Tick or Vicia faba – 500 1000 500 Pea” leguminosarum Broad bean bv. viciae Pea, Field pea Pisum sativum Common Vicia sativa – 500 1000 500 Vetch or Tare Bitter vetch V. ervilia Lathyrus Lathyruscicera Purple vetch V. benghalensis Woolly Pod V. villosa spp. vetch dasycarpa Lentil Lens culinaris – 250 500 250 G “Lupin” (WU425) Bradyrhizobium Narrow leaf Lupinus – 500 1000 500 sp. lupin angustifolius Mediterranean L. albus white lupin Yellow lupin L. -

Centrosema Pascuorum Cv
African Journal of Biotechnology Vol. 11(92), pp. 15843-15851, 15 November, 2012 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB11.2412 ISSN 1684–5315 ©2012 Academic Journals Full Length Research Paper Variation and long term regenerative capacity of two important tropical forage legumes: Cavalcade (Centrosema pascuorum cv. Cavalcade) and Stylo 184 (Stylosanthes guianensis CIAT184) in vitro Varaporn Veraplakorn1, Malee Na Nakorn2, Lily Kaveeta2, Srisom Suwanwong2 and Ian James Bennett3* 1Department of Biotechnology, Faculty of Science, Ramkhamhaeng University, Bangkok 10240, Thailand. 2Department of Botany, Faculty of Science, Kasetsart University, Bangkok 10900, Thailand. 3School of Natural Sciences, Edith Cowan University, Western Australia. Accepted 27 July, 2012 Shoots of Cavalcade (Centrosema pascuorum cv. Cavalcade) and Stylo 184 (Stylosanthes guianensis CIAT 184) from in vitro germinated seeds were cultured on Murashige and Skoog (MS) medium supplemented with 0 to 7 mg L-1 N 6-benzyladenine (BA) in combination with 0 to 0.5 mg L-1 napthalene acetic acid (NAA) for shoot induction and MS supplemented with 0 to 0.5 mg L-1 indolebutyric acid (IBA) for root induction. For Cavalcade, the medium containing 1 mg L-1 BA produced the best shoot multiplication with an excess of six shoots produced from a single shoot (over four weeks) with a mean height 2.0 ± 0.01 cm. Adventitious shoot regeneration was obtained directly from stem axes. For Stylo 184, the maximum shoot regeneration (29.5 ± 1.0 cm shoots/explant) and height (1.5 ± 0.1 cm) was achieved using 7 mg L-1 BA and 0.01 mg L-1 NAA. -

Final Report Development of Low Input Systems Such As Organic Farming By
Final report Development of low input systems such as organic farming by optimising the use of legumes in a dry region of Nicaragua to strengthen soil fertility, yield, human nutrition and farm income Use of legumes in low input systems ULLIS Glenda Bonilla (MIS/UNA) Emilio Perez (UNA) Carlos Ruiz (UNA) Rein van der Hoek (CIAT) Diana Kurzweg (BOKU) Bernhard Freyer (BOKU) University of Natural Resources and Applied Life Sciences (BOKU) Universidad Nacional Agraria (MIS/UNA) Centro Internacional de Agricultura Tropical (CIAT) Table of contents Acknowledgements ........................................................................................................................ 1 Abstract .......................................................................................................................................... 2 Zusammenfassung ......................................................................................................................... 3 1. Introduction ............................................................................................................................... 4 2. Objectives ................................................................................................................................... 5 3. Activities February 2005 – September 2007 ........................................................................... 6 3.1 Evaluation of the adaptability of 16 genotypes of forage legumes in the sub-humid zone of San Dionisio, Matagalpa ................................................................................................. -

Pharmacological Importance of Clitoria Ternatea – a Review
IOSR Journal Of Pharmacy www.iosrphr.org (e)-ISSN: 2250-3013, (p)-ISSN: 2319-4219 Volume 6, Issue 3 (March 2016), PP. 68-83 Pharmacological importance of Clitoria ternatea – A review Prof Dr Ali Esmail Al-Snafi Department of Pharmacology, College of Medicine, Thi qar University, Nasiriyah, Iraq. Abstract: Clitoria ternatea contained tannins, phlobatannin, carbohydrates, saponins, triterpenoids, phenols, flavanoids, flavonol glycosides, proteins, alkaloids, antharaquinone, anthocyanins, cardiac glycosides, Stigmast- 4-ene-3,6-dione, volatile oils and steroids. The plant showed many pharmacological effects including antioxidant, hypolipidemic, anticancer, anti-inflammatory, analgesic, antipyretic, antidiabetic, CNS, antimicrobial, gastro-intestinal antiparasitic, insecticidal and many other pharmacological effects. This Review will highlight the chemical constituents and pharmacological effects of Clitoria ternatea. Keywords: Clitoria ternatea, constituents, pharmacology, pharmacognosy I. INTRODUCTION A large and increasing number of patients in the world use medicinal plants and herbs for health purpose. Therefore, scientific scrutiny of their therapeutic potential, biological properties, and safety will be useful in making wise decisions about their use(1-2). There are hundreds of significant drugs and biologically active compounds developed from the traditional medicinal plants. Plant showed wide range of pharmacological activities including antimicrobial, antioxidant, anticancer, hypolipidemic, cardiovascular, central nervous, respiratory, -

Karyomorphological Studies on Seven Variants of Clitoria Terantea L. (Fabaceae)
ISSN (Online): 2349 -1183; ISSN (Print): 2349 -9265 TROPICAL PLANT RESEARCH 6(2): 320–325, 2019 The Journal of the Society for Tropical Plant Research DOI: 10.22271/tpr.2019.v6.i2.041 Research article Karyomorphological studies on seven variants of Clitoria terantea L. (Fabaceae) J. Shamnad* and Dan Mathew Plant Genetic Resource Division, Jawaharlal Nehru Tropical Botanic Garden and Research Institute, Palode, Thiruvananthapuram, Kerala, India *Corresponding Author: [email protected] [Accepted: 25 August 2019] Abstract: Mitosis of seven variants of Clitoria ternatea was carried out and the number of chromosomes in all the variants were 16. Karyotype indicated that all the variants consist of nearly median and sub-median chromosomes. Total and absolute chromosome length were higher in double pink and lower in light blue. The analysis of total form percentage showed that ‛double pink’ possess lowest value and represented as the most advanced type whereas ‛single blue’ possess highest value and represented as the most primitive karyotype. The variation in chromosomal characters coincides with the morphological variability within the species. Keywords: Karyotype - Butterfly pea - Asymmetrical - Idiogram. [Cite as: Shamnad J & Mathew D (2019) Karyomorphological studies on seven variants of Clitoria terantea L. (Fabaceae). Tropical Plant Research 6(2): 320–325] INTRODUCTION Clitoria ternatea L. commonly known as ‛Butterfly Pea’ (Shankupushpam) belongs to the tribe Phaseolae of the family Fabaceae. It is probably a native to South America (Upadhyaya & Pachauri 1983, Parrotta 2001, Harinarayanan 2005) and widely distributed in tropical and sub-tropical countries. The plant is a perennial, tall and branched with white to blue/pinkish flowers. -

Clitoria Ternatea Scientific Name Clitoria Ternatea L
Tropical Forages Clitoria ternatea Scientific name Clitoria ternatea L. Synonyms Common blue flowered form; imparipinnate leaves (P 16020) Persistent multi-purpose herbaceous Clitoria albiflora Mattei; Clitoria bracteata Poir.; Clitoria perennial suitable for groundcover, mearnsii De Wild.; Clitoria tanganicensis Micheli; green manure, protein bank etc. Clitoria zanzibarensis Vatke Family/tribe Family: Fabaceae (alt. Leguminosae) subfamily: Faboideae tribe: Phaseoleae subtribe: Clitoriinae. Morphological description Perennial climbing, scrambling or trailing herb with a Mauve flowered form Erect climbing, scrambling or trailing strong, woody rootstock. Main stem suberect to erect, perennial. Image: sown into crop somewhat woody at base; secondary stems fine, twining, stubble. sparsely pubescent or glabrescent, 0.5‒3 m long. Leaves imparipinnate with 2‒3 (‒4) pairs of leaflets and a terminal leaflet; petiole 1.5‒3 cm long; stipules persistent, narrowly triangular, (1‒) 2‒5 (‒6) mm long, subulate, prominently 3-nerved; rachis 1‒7 cm long; stipels filiform, to 2 mm long; petiolules 1‒2 mm long; leaflets elliptic, ovate or nearly orbicular, 1.5‒5 cm long, 0.3‒3 cm wide, apex acute or rounded, often notched (emarginate), usually mucronate; base cuneate Mature and immature pods or rounded; both surfaces sparsely appressed pubescent, sometimes glabrous. Flowers axillary, single or paired; pedicles 4‒9 mm long, resupinate; bracteoles White flowered form persistent, 4‒12 mm long, broadly ovate or rounded with obvious reticulate veins; calyx 5-lobed, lobes lanceolate, 1.5‒2.2 cm long with a few fine hairs; corolla white, pink, mauve, light blue to dark blue; tube campanulate, 0.8‒1.2 cm long; lobes triangular or oblong, 0.7‒1 cm long, acute or acuminate; standard obovate, funnel-shaped, 2‒5.5 cm long, 2‒4 cm wide, notched or rounded at apex, white and pale yellowish-green centre, a few fine hairs at apex; wings and keels much shorter than standard. -

The Ayurvedic Pharmacopoeia of India; Part – I Volume
THE AYURVEDIC PHARMACOPOEIA OF INDIA PART- I VOLUME – II GOVERNMENT OF INDIA MINISTRY OF HEALTH AND FAMILY WELFARE DEPARTMENT OF AYUSH Contents | Monographs | Abbrevations | Appendices Legal Notices | General Notices Note: This e-Book contains Computer Database generated Monographs which are reproduced from official publication. The order of contents under the sections of Synonyms, Rasa, Guna, Virya, Vipaka, Karma, Formulations, Therapeutic uses may be shuffled, but the contents are same from the original source. However, in case of doubt, the user is advised to refer the official book. i CONTENTS Legal Notices General Notices MONOGRAPHS S No. Plant Name Botanical Name Page No. (as per book) 1 ËKËRAKARABHA (Root) Anacyclus pyrethrum DC 1 2 AKâOÚA (Cotyledon) Juglans regia Linn 3 3 ËMRËTA (Stem Bark) Spondias pinnata Linn.f.Kurz. 5 4 APËMËRGA (Whole Plant) Achyranthes aspera Linn. 7 5 APARËJITË (Root) Clitoria ternatea Linn 10 6 ËRDRAKA (Rhizome) Zingiber officinale Rosc 12 7 ARIMEDA (Stem Bark) Acacia leucophloea Willd. 15 8 ARJUNA (Stem Bark) Terminalia arjuna W& A. 17 9 BHALLËTAKA (Fruit) Semecarpus anacardium Linn 19 10 BHÎ×GARËJA (Whole Plant) Eclipta alba Hassk 21 11 BRËHMÌ (Whole Plant) Bacopa monnieri (Linn.) Wettst. 25 12 12. BÎHATÌ (Root) Solanum indicum Linn 27 13 CAVYA (Stem) Piper retrofractum Vahl. 29 14 DËÚIMA (Seed) Punica granatum Linn 31 15 DËRUHARIDRË (Stem) Berberis aristata DC 33 16 DROÛAPUâPÌ (Whole Plant) Leucas cephalotes Spreng. 35 17 ERVËRU (Seed) Cucumis melo var utlissimus Duthie 38 & Fuller 18 GAJAPIPPALÌ (Fruit) Scindapsus officinalis Schooott 40 19 GAMBHARI (Fruit) Gmelina arborea Roxb 42 20 GË×GERU (Stem bark) Grewia tenax (Forsk.) Aschers & 44 Schwf. -

Foraging Koh
Foraging Guide Belimbing Betel Butterfly Pea Flower Averrhoa bilimbi Piper Betle Clitoria ternatea L. Flowers, fruits are sour but Eaten with the betel nut (Areca Medicinal: Swelling, sore piquant. Eaten raw or in a catechu) as a stimulant. throat, etc. Also lends pickle! Leaves are medicinal - contain materials a natural blue antimicrobial and antioxidant colouring. https:// activity. florafaunaweb.nparks.gov.sg/ https:// Special-Pages/plant-detail.aspx? https:// florafaunaweb.nparks.gov.sg/ id=2735 florafaunaweb.nparks.gov.sg/ Special-Pages/plant-detail.aspx? special-pages/plant-detail.aspx? id=1372 id=1490 ! Cashew Asiatic Pennywort Cempedak Anacardium occidentale Centella Asiatica Artocarpus integer (Thunb.) Cashew apple is normally Entire plant can be used for Merr. eaten, and can be made into a healing of wounds, burns, etc. jam or seeetmeat. Great for Juice is drunk for cooling Not to be confused with fermentation. The bark and nut properties. jackfruit, the fruits and leaves oil can be used to treat corns, are eaten - seeds can also be https:// warts, ulcers. eaten after roasting or boiled. florafaunaweb.nparks.gov.sg/ Special-Pages/plant-detail.aspx? https:// id=5347 https:// florafaunaweb.nparks.gov.sg/ florafaunaweb.nparks.gov.sg/ Special-Pages/plant-detail.aspx? Special-Pages/plant-detail.aspx? id=2709 id=3309 ! ! Chinese Violet Citronella Climbing Wattle Asystasia gangetica ssp. Cymbopogon nardus Acacia Pennata L micrantha (Nees) Ensermu Leaves, stem and flower are Not to be confused with Known as cha - om in Thai, a eaten raw or blanched, or in a lemongrass, is used to legume frequently found in stirfry. Crunchy in texture.